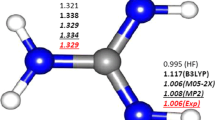
Abstract
The geometrical structure and binding energy of small clusters of methyl radical and water molecules (up to five water molecules) in gas phase and water media have been investigated at the MP2 level of theory using 6-311++G(2df,2p) basis set. The complexes characterized contain OH···O, CH···O, and OH···C attractive interactions with stabilization energies in the range 6–143 kJ mol−1. The solvent has an enhancing influence on the stabilities of studied clusters. The atoms in molecules theory were also applied to explain the nature of the complexes. The interaction energies have been partitioned with the natural energy decomposition analysis showing that the most important attractive term corresponds to the charge transfer one.



Similar content being viewed by others
References
Muller-Dethlefs K, Hobza P (2000) Chem Rev 100:143
Scheiner S (1997) Hydrogen bonding. A theoretical perspective. Oxford University Press, Oxford and references therein
Hobza P, Havlas Z (2000) Chem Rev 100:4253
Solimannejad M, Azimi G, Lj Pejov (2004) Chem Phys Lett 400:185
Solimannejad M, Azimi G, Pejov Lj (2004) Chem Phys Lett 391:201
Solimannejad M, Alikhani ME (2005) Chem Phys Lett 406:351
Solimannejad M, Alkorta I (2006) J Phys Chem A 110:10817
Solimannejad M, Scheiner S (2006) Chem Phys Lett 429:38
Solimannejad M, Scheiner S (2006) J Phys Chem A 110:5948
Solimannejad M, Shirazi SG, Scheiner S (2007) J Phys Chem A 111:10717
Solimannejad M, Nielsen CJ, Scheiner S (2008) Chem Phys Lett 466:136
Solimannejad M, Massahi S, Scheiner S (2009) J Mol Struct (THEOCHEM) 913:50
Solimannejad M, Scheiner S (2009) Mol Phys 107:713
Johnson ER, Dilabio GA (2009) Interdiscip Sci 1:133
Hammerum S (2009) J Am Chem Soc 131:8627
Franchi P, Lucarini M, Pedrielli P, Pedulli GF (2002) Chem Phys Chem 3:789
Alkorta I, Rozas J, Elguero J (1998) J Phys Chem 102:429
Wang B, Li Z, Wu D, Hao X, Li R, Sun C (2003) Chem Phys Lett 375:91
Tang K, Shi FQ (2007) Int J Quantum Chem 107:665
An X, Liu H, Li Q, Gong B, Cheng J (2008) J Phys Chem A 112:5258
Li Q, Zhu H, An X, Gong B (2009) Int J Quantum Chem 109:605
Romieu A, Bellon S, Gasparutto D (2000) Org Lett 2:1085
Alkorta I, Elguero J (2003) ARKIVOC xiv:31
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) GAUSSIAN03, Revision B.03. Gaussian, Inc, Pittsburgh, PA
Qi XJ, Liu L, Fu Y, Guo QX (2005) Struct Chem 16:347
Boys SF, Bernardi F (1970) Mol Phys 19:553
Alkorta I, Trujillo C, Elguero J, Solimannejad M (2011) Comput Theor Chem 967:147
Bader RFW (1990) In: Halpen J, Green MLH (eds) The international series of monographs of chemistry. Clarendon Press, Oxford
Biegler-Konig F, Schonbohm J (2002) AIM2000 Program Package, Ver. 2.0. University of Applied Sciences, Bielefeld
Glendening ED (2011) J Am Chem Soc 118:2473
Glendening ED (2005) J Phys Chem A 109:11936
Weinhold F, Landis CR (2005) Valency and bonding. A natural bond orbital donor–acceptor perspective. Cambridge Press, Cambridge
Zhao Y, Truhlar DG (2006) J Chem Theory Comput 2:1009
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JE, Morales CM, Weinhold F (2004) NBO 5.0. Theoretical Chemistry Institute, University of Wisconsin, Madison, WI
Schmidt JW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347
Tomasi J, Mennucci B, Cammi R (1993) Chem Rev 105:2999
Shields RM, Temelso B, Archer KA, Morrell TE, Shields GC (2010) J Phys Chem A 114:11725
Ugalde JM, Alkorta I, Elguero J (2000) Angew Chem Int Ed 39:717
Gregory JK, Clary DC (1996) J Phys Chem 100:18014
Alkorta I, Blanco F, Deya PM, Elguero J, Estarellas C, Frontera A, Quinonero D (2010) Theor Chem Acc 126:1
Rozas I, Alkorta I, Elguero J (2000) J Am Chem Soc 122:11154
Mata I, Alkorta I, Molins E, Espinosa E (2010) Chem Eur J 16:2442
Alkorta I, Zborowski K, Elguero J, Solimannejad M (2006) J Phys Chem A 110:10279
Li QZ, Wang NN, Yu ZW (2007) J Mol Struct (THEOCHEM) 847:68
Li QZ, An XL, Gong BA, Cheng JB (2007) J Phys Chem A 111:10166
Acknowledgments
This work was carried out with financial support from Arak University (Grant No. 90.12650).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Solimannejad, M., Gharabaghi, M. & Alkorta, I. Ab initio study of water clustering in the presence of a methyl radical. Struct Chem 24, 491–497 (2013). https://doi.org/10.1007/s11224-012-0099-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-012-0099-7