Abstract
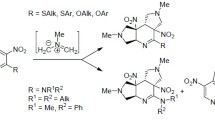
[6+4] Cycloaddition reactions of functionalized thiophene dioxides with various fulvenes provided the corresponding azulenes. The observed regiochemistry was explained in terms of the HOMO—LUMO orbital coefficients.
Similar content being viewed by others
References
K.-P, Zeller, in Houben-Weyl; Methoden der organischen Chemie, Georg Thieme, Stuttgart, 1985; Vol. V/2c, 127 pp.
T. Zieliñski, M. Kedziorek, and J. Jurczak, Tetrahedron Lett., 2005, 46, 6231; (b) M. Lamberto, C. Pagba, P. Piotrowiak, and E. Galoppini, Tetrahedron Lett., 2005, 46, 4895.
S. Ito, S. Kikuchi, N. Morita, and T. Asao, J. Org. Chem., 1999, 64, 5815.
T. Asao, S. Ito, and N. Morita, Tetrahedron Lett., 1988, 29, 2839; (b) D. A. Colby and T. D. Lash, J. Org. Chem., 2002, 67, 1031; (c) O. K. Abou-Zied, Spectrochim. Acta, Part A, 2005, 62, 245.
F. A. Andersen and D. R. Teufel, Int. J. Toxicol., 1999, 18, 27; (b) M. Guarrera, L. Turbino, and A. Rebora, J. Eur. Acad. Dermatol. Venerol., 2001, 15, 486.
K. Ziegler and K. Hafner, Angew. Chem., 1955, 67, 301; (b) L. T. Scott, M. A. Minton, and M. A. Kirms, J. Am. Chem. Soc., 1980, 102, 6311; (c) M. Yasunami, S. Miyoshi, N. Kanegae, and K. Takase, Bull. Chem. Soc. Jpn, 1993, 66, 892; (d) S. Carret, A. Blanc, Y. Coquerel, M. Berthod, A. E. Greene, and J.-P. Deprés, Angew. Chem., Int. Ed. (Engl.), 2005, 44, 5130.
V. G. Nenajdenko, A. E. Gavryushin, and E. S. Balenkova, Tetrahedron Lett., 2001, 42, 4397; (b) V. G. Nenajdenko, A. M. Moiseev, and E. S. Balenkova, Izv. Akad. Nauk, Ser. Khim., 2004, 2144 [Russ. Chem. Bull., Int. Ed., 2004, 53, 2241].
V. G. Nenajdenko, A. M. Moiseev, and E. S. Balenkova, Tetrahedron, 2005, 61, 10880; (b) V. G. Nenajdenko, A. M. Moiseev, and E. S. Balenkova, Izv. Akad. Nauk, Ser. Khim., 2005, 2117 [Russ. Chem. Bull., Int. Ed., 2005, 54, 2182]; (c) A. M. Moiseev, V. G. Nenajdenko, and E. S. Balenkova, Tetrahedron, 2006, 61, 4139.
S. E. Reiter, L. C. Dunn, and K. N. Houk, J. Am. Chem. Soc., 1977, 99, 4199; (b) D. Mukherjee, L. C. Dunn, and K. N. Houk, J. Am. Chem. Soc., 1979, 101, 251; (c) K. Kanematsu, H. Kazunobu, and D. Hirokazu, Heterocycles, 1981, 16, 1145; (d) Y. Lu, D. M. Lemal, and J. P. Jasinski, J. Am. Chem. Soc., 2000, 122, 2440; (e) J. Nakayama, Bull. Soc. Chem. Jpn, 2000, 73, 1; (f) Y. Lou, J. Chang, J. Jorgensen, and D. M. Lemal, J. Am. Chem. Soc., 2002, 124, 15302.
K. Hafner, K. H. Vöpel, G. Ploss, and C. König, Organic Syntheses, John Wiley & Sons, New York, 1973, Coll. Vol. 5, p. 431; (b) P. Rademacher, R. Stolevik, and W. Lüttke, Angew. Chem., Int. Ed. (Engl.), 1968, 10, 806; (c) N. F. Bondar’, E. V. Koroleva, T. N. Omel’chenko, and A. A. Akhrem, Zh. Org. Khim., 1981, 17, 2333 [J. Org. Chem. USSR, 1981, 17, 2084]; (d) I. Gupta and P. Yates, Synth. Commun., 1982, 13, 1007; (e) G. Nzabamwita, B. Kolani, and B. Jousseaume, Tetrahedron Lett., 1989, 30, 2207.
K. N. Houk, J. K. George, and R. E. Duke, Tetrahedron, 1974, 30, 523; (b) K. N. Houk, J. Sims, C. R. Watts, and L. J. Luskus, J. Am. Chem. Soc., 1973, 95, 7301.
Author information
Authors and Affiliations
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 1, pp. 137–142, January, 2006.
Rights and permissions
About this article
Cite this article
Moiseev, A.M., Balenkova, E.S. & Nenajdenko, V.G. [6+4] Cycloaddition reactions of acceptor thiophene dioxides: The synthesis of substituted azulenes. Russ Chem Bull 55, 141–146 (2006). https://doi.org/10.1007/s11172-006-0227-x
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11172-006-0227-x