Abstract
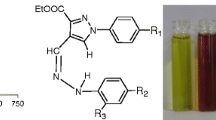
In this paper, a new ferrocenylimidazole compound L was designed and synthesized. L could selectively sense a fluoride anion among test anions such as F−, AcO−, Cl−, Br−, I− and HSO4 −, accompanied by obvious color or electrochemical changes. The detection limit in a DMSO solution was calculated as 5.94 × 10−6 M−1. From the Uv–vis, NMR and electrochemical titrations, it was indicated that L and fluoride anions formed a 1:1 complex with hydrogen bonding and anion–π interactions.








Similar content being viewed by others
References
R. Martinez-Mannez, F. Sancenon, Chem. Rev. 4419, 103 (2003)
M. Cametti, K. Rissanen, Chem. Commun. 2809, 20 (2009)
M. Yousuf, N. Ahmed, B. Shirinfar et al., Org. Lett. 2150, 16 (2014)
X.D. Yu, Y.J. Li, Y.B. Yin, D.C. Yu, Mater. Sci. Eng., C 1695, 32 (2012)
L.M. Salonen, M. Ellermann, F. Diederich et al., Angew. Chem. Int. Ed. 4808, 50 (2011)
O. Perraud, V. Robert, H. Gornitzka et al., Angew. Chem. Int. Ed. 504, 51 (2012)
N. Ahmed, B. Shirinfar, V.M. Miriyala et al., Supramol. Chem. 478, 27 (2015)
A. Tarraga, P. Molina, Inorg. Chem. 7487, 52 (2013)
Z. Xu, X. Chen, H.N. Kim, J. Yoon, Chem. Soc. Rev. 127, 39 (2010)
C. Caltagirone, P.A. Gale, Chem. Soc. Rev. 520, 38 (2009)
X.D. Yu, P. Zhang, Q. Liu, Y. Li, X. Zhen, Y. Zhang, Z. Ma, Mater. Sci. Eng., C 73, 39 (2014)
X.D. Yu, P. Zhang, Y. Li, X.L. Zhen, L. Geng, Y. Wang, Z. Ma, Mater. Sci. Eng. C 467, 40 (2014)
M.X.D. Yu, H. Lin, Z. Cai, H. Lin, Tetrahedron Lett. 8615, 48 (2007). (Alfonso)
S.K. Kim, J.L. Sessler, Chem. Soc. Rev. 3784, 39 (2010)
M. Alfonso, A. Tarraga, P. Molina, Inorg. Chem. 7487, 52 (2013)
Q. Tan, L. Wang, L. Ma, H. Yu, J. Ding, Q. Liu, A. Xiao, G. Ren, J. Phys. Chem. B 11171, 112 (2008)
X. Ming Liu, Q. Zhao, Y. Li, W.O. Song, Y. Li, Z. Chang, X. Bu, Chin. Chem. Lett. 962, 24 (2013)
K. Flídrová, M. Tkadlecová, K. Lang, P. Lhoták, Tetrahedron Lett. 678, 53 (2012)
D. Lee, H.Y. Lee, K.H. Lee, J. Hong, Chem. Commun. 1188, 13 (2001)
M. Zora, A. Kivrak, Y. Kelgokmen, J. Organomet. Chem. 67, 759 (2014)
R. Maragani, R. Misra, Tetrahedron Lett. 5399, 39 (2013)
B. Shirinfar, N. Ahmed, Y.S. Park et al., J. Am. Chem. Soc. 90, 135 (2013)
N. Ahmed, B. Shirinfar II, S. Youn et al., Org. Biomol. Chem. 6407, 11 (2013)
A. Frontera, P. Gamez, M. Mascal et al., Angew. Chem. Int. Ed. 9564, 50 (2011)
Acknowledgments
Yu et al. gratefully acknowledge Prof. Tao Yi in Fudan chemistry for the partial experiment support and research fellowships. This work was supported by the National Natural Science Foundation of China (No. 21301047), Natural Science Foundation of Hebei Province (No. B2014208094).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Yu, X. Colorimetric and electrochemical sensing for fluoride anion by ferrocenyl-based imidazole compound with electron donor–acceptor structure. Res Chem Intermed 43, 1099–1105 (2017). https://doi.org/10.1007/s11164-016-2685-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2685-6