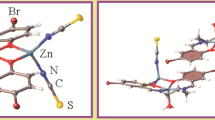
Abstract
A new mononuclear Zn(II) complex, [Zn(L 2 )2]·CH3OH (HL 2 = 1-(2-{[(E)-3,5-dichloro-2-hydroxybenzylidene]amino}phenyl)ethanone oxime), has been synthesized via complexation of Zn(II) acetate dihydrate with HL 1 (HL 1 = 2-(3,5-dichloro-2-hydroxyphenyl)-4-methyl-1,2-dihydroquinazoline 3-oxide) originally. HL 1 and its corresponding Zn(II) complex were characterized by infrared (IR), ultraviolet-visible light (UV–Vis) and emission spectroscopy, as well as by elemental analysis. The crystal structure of the complex has been determined by single-crystal X-ray diffraction (XRD). Each complex links two other molecules into an infinite one-dimensional (1-D) chain through intermolecular hydrogen bonds. Moreover, the calculated highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies show the character of the ligand HL 1 and the Zn(II) complex. Time-dependent density functional theory (TDDFT) calculations were done on the optimised geometries to understand the electronic structure and spectral transition in the ligand and the Zn(II) complex.
Graphical abstract
A new mononuclear Zn(II) complex involving a Schiff base-type instead of an anticipated quinazoline complex has been synthesized and characterized structurally by spectroscopic methods. The crystal structure of the complex has been determined by single-crystal XRD. Each complex links two other molecules into an infinite 1-D chain through intermolecular hydrogen bonds. Moreover, the calculated HOMO and LUMO energies show the character of the ligand HL 1 and the Zn(II) complex. The TDDFT calculations were done on the optimised geometries to understand the electronic structure and spectral transition in the ligand and the Zn(II) complex.










Similar content being viewed by others
References
H. Furukawa, K.E. Cordova, M. O'Keeffe, O.M. Yaghi, Science 341, 974–986 (2013)
Z.H. Chohan, S.H. Sumrra, M.H. Youssoufi, T.B. Hadda, Eur. J. Med. Chem. 45, 2739–2747 (2010)
H. Wu, D.F. Wang, J. Shi, S. Xue, M.L. Gao, J. Agric. Food Chem. 58, 5757–5762 (2010)
L.F. Ma, C.P. Li, L.Y. Wang, M. Du, Cryst. Growth Des. 11, 3309–3312 (2011)
X.Y. Zhou, B.R. Yu, Y.L. Guo, X.L. Tang, H.H. Zhang, W.S. Liu, Inorg. Chem. 49, 4002–4007 (2010)
A.I. Sachin, S. Frank, J. Org. Chem. 77, 9352–9356 (2012)
M.V. Gastel, C.C. Lu, K. Wieghardt, W. Lubitz, Inorg. Chem. 48, 2626–2632 (2009)
Z.J. Zhang, P. Cui, X.Y. Chen, Ind. Eng. Chem. Res. 52, 16211–16219 (2013)
W.S. Xia, C.H. Huang, D.J. Zhou, Langmuir 13, 80–84 (1997)
S.T. Zhang, T.R. Li, B.D. Wang, Z.Y. Yang, J. Liu, Z.Y. Wang, W.K. Dong, Dalton Trans. 43, 2713–2717 (2014)
W. Maret, Y. Li, Chem. Rev. 109, 4682–4707 (2009)
I.M.A. Mundo, K.E. Siters, M.A. Fountain, J.R. Morrow, Inorg. Chem. 51, 5444–5457 (2012)
H. Park, K.M. Merz Jr, J. Med. Chem. 48, 1630–1637 (2005)
B.X. Wei, A.M. Randich, M.B. Pakrasi, H.B. Pakrasi, T.J. Smith, Biochemistry 46, 8734–8743 (2007)
R. Alonso, A. Caballero, P.J. Campos, D. Sampedro, M.A. Rodríguez, Tetrahedron 66, 4469–4473 (2010)
E.M. Olasik, K.B. Światkiewiz, E. Żurek, U. Krajewska, M. Różalski, T.J. Bartczak, Arch. Pharm. Med. Chem. 337, 239–246 (2004)
J.M. Xiao, W. Zhang, Inorg. Chem. Commun. 12, 1175–1178 (2009)
M. Kalanithi, M. Rajarajan, P. Tharmara, J. Coord. Chem. 64, 842–850 (2011)
L.Q. Chai, G. Liu, Y.L. Zhang, J.J. Huang, J.F. Tong, J. Coord. Chem. 66, 3926–3938 (2013)
L.Q. Chai, H.S. Zhang, J.J. Huang, Y.L. Zhang, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 137, 661–669 (2015)
L.Q. Chai, J.J. Huang, J.Y. Zhang, Y.X. Li, J. Coord. Chem. 68, 1224–1237 (2015)
L.Q. Chai, Y.L. Zhang, K. Cui, Z.R. Wang, L.W. Zhang, Y.Z. Zhang, Z. Kristallogr, New. Cryst. Struct. 227, 153–154 (2012)
G.W.T.M.J. Frisch, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, O.Y. Stratmann, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian Inc., Gaussian 09, Revision A. 01 ed, (Wallingford, CT 2009). http://refhub.elsevier.com/S0925-4005(15)00769-8/sbref0210
A.D. Becke, J. Chem. Phys. 98, 5648–5652 (1993)
C. Lee, W. Yang, R.G. Parr, Phys. Rev. B 37, 785–789 (1988)
P.J. Hay, W.R. Wadt, J. Chem. Phys. 82, 270–283 (1985)
W.R. Wadt, P.J. Hay, J. Chem. Phys. 82, 284–298 (1985)
P.J. Hay, W.R. Wadt, J. Chem. Phys. 82, 299–310 (1985)
R. Bauernschmitt, R. Ahlrichs, Chem. Phys. Lett. 256, 454–464 (1996)
R.E. Stratmann, G.E. Scuseria, M.J. Frisch, J. Chem. Phys. 108, 8218–8224 (1998)
M.E. Casida, C. Jamorski, K.C. Casida, D.R. Salahub, J. Chem. Phys. 108, 4439–4449 (1998)
V. Barone, M. Cossi, J. Phys. Chem. A 102, 1995–2001 (1998)
M. Cossi, V. Barone, J. Chem. Phys. 115, 4708–4717 (2001)
M. Cossi, N. Rega, G. Scalmani, V. Barone, J. Comput. Chem. 24, 669–681 (2003)
T. Lu, F.W. Chen, J. Comp. Chem. 33, 580–592 (2012)
T. Lu, F.W. Chen, J. Mol. Graph. Model. 38, 314–323 (2012)
G.M. Sheldrick, Acta Crystallogr. A 64, 112–122 (2008)
G.M. Sheldrick, SHELXS-97 and SHELXL-97, Program for the Refinement of Crystal Structures (University of Göttingen, Germany, 1997)
M.M. Carthy, P.J. Gyiry, Polyhedron 19, 541–543 (2000)
K. Das, A. Jana, S. Konar, S. Chatterjee, T.K. Mondal, A.K. Barik, S.K. Kar, J. Mol. Struct. 1048, 98–107 (2013)
D. Kovala-Demertzi, V.N. Dokorou, J.P. Jasinski, A. Opolski, J. Wiecek, M. Zervou, M.A. Demertzis, J. Org. Chem. 690, 1800–1806 (2005)
D. Hauchecorne, N. Nagels, B.J. van der Veken, W.A. Herrebout, Phys. Chem. Chem. Phys. 14, 681–690 (2012)
L.Q. Chai, J.J. Huang, H.S. Zhang, Y.L. Zhang, J.Y. Zhang, Y.X. Li, Spectrochim. Acta Part A: Mol. Biomol. Spect. 131, 526–533 (2014)
T.Z. Yu, K. Zhang, Y.L. Zhao, C.H. Yang, H. Zhang, L. Qian, D.W. Fan, W.K. Dong, L.L. Chen, Y.Q. Qiu, Inorg. Chim. Acta 361, 233–240 (2008)
C.J. Dhanaraj, J. Johnson, J. Joseph, R.S. Joseyphus, J. Coord. Chem. 66, 1416–1450 (2013)
M.K. Paria, J. Dinda, T.H. Lu, A.R. Paital, C. Sinha, Polyhedron 26, 4131–4140 (2007)
T. Ghosh, B. Mondal, T. Ghosh, M. Sutradhar, G. Mukherjee, M.G.B. Drew, Inorg. Chim. Acta 360, 1753–1761 (2007)
W.K. Dong, S.J. Xing, Y.X. Sun, L. Zhao, L. Q. Chai, X. H. Gao. J. Coord. Chem. 65, 1212–1220 (2012)
S. Mandala, S. Chatterjeeb, R. Modaka, Y. Sikdara, B. Naskara, S. Goswamia, J. Coord. Chem. 67, 699–713 (2014)
Y.H. Zhou, D.L. Sun, J. Tao, L.Q. Chen, Y.F. Huang, Y.K. Lia, Y. Cheng, J. Coord. Chem. 67, 2393–2404 (2014)
Y.H. Zhou, W.Q. Wan, D.L. Sun, J. Tao, L. Zhang, X.W. Wei, Z. Anorg, Allg. Chem. 640, 249–253 (2014)
Acknowledgments
We are thankful for the financial support by the Fundamental Research Funds for the Universities of Gansu Province (No. 2140152).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chai, LQ., Zhang, JY., Chen, LC. et al. Synthesis, crystal structure, spectroscopic properties and DFT calculations of a new Schiff base-type Zinc(II) complex. Res Chem Intermed 42, 3473–3488 (2016). https://doi.org/10.1007/s11164-015-2226-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2226-8