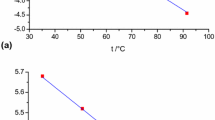
Abstract
With the calorimetric (adsorption heat versus coverage) curve also measured together with the adsorption isotherm, the simultaneous use of both curves showed that there were two phases of adsorption in the adsorption of methanol, dimethyl ether, ethene and propane in SAPO-34. The dual-site Langmuir equation gave good fits to the adsorption data to support the interpretation that a second (type 2) adsorption phase occurred in the high-pressure region in addition to a first (type 1) adsorption phase on the acid sites at lower pressures. Adsorption experiments and calculations using binary gas mixtures showed that due to the existence of two types of adsorption, the multicomponent Langmuir isotherm equation (Langmuir competitive adsorption model) calculated incorrect surface concentrations when the concentrations were high. In contrast, the ideal adsorbed solution theory (IAST) calculated correct surface concentrations in the adsorption of mixtures.







Similar content being viewed by others
References
H.S. Fogler, Elements of Chemical Reaction Engineering, 4th edn. (Prentice-Hall, New Jersey, 1999), pp. 594–600
G.F. Froment, K.B. Bischoff, J. De Wilde, Chemical Reactor Analysis and Design, 3rd edn. (Wiley, New Jersey, 2011), pp. 69–71
M.A. Vannice, Kinetics of Catalytic Reactions (Springer, New York, 2005), pp. 91–97
D.D. Do, Adsorption Analysis: Equilibria and Kinetics (Imperial College Press, London, 1998), pp. 191–248
A.L. Myers, J.M. Prausnitz, AIChE J. 11, 121 (1965)
T. Titze, C. Chmelik, J. Karger, J.M. van Baten, R. Krishna, J. Phy, Chem. C 118, 2660 (2014)
N. Hansen, R. Krishna, J.M. van Baten, A.T. Bell, F.J. Keil, J. Phy, Chem. C 113, 235 (2009)
T. Remy, J.C. Saint Remi, R. Singh, P.A. Webley, G.V. Baron, J.F.M. Denayer, J. Phys. Chem. C 115, 8117 (2011)
B.M. Lok, C.A. Messina, R.L. Patton, R.T. Gajek, T.R. Cannan, E.M. Flanigen, US Patent 4440871, to Union Carbide Corporation (1984)
Y.X. Li, M.Y. Zhang, D.Z. Wang, F. Wei, Y. Wang, J. Catal. 311, 281 (2014)
Y. Kobayashi, Y.X. Li, Y. Wang, D.Z. Wang, Chin. J. Catal. 35, 430 (2014)
Y. Kobayashi, F. Wang, Q.X. Li, D.Z. Wang, Rev. Sci. Instrum. 85, 34101 (2014)
D.M. Ruthven, in Molecular Sieves, Science and Technology, vol. 7, ed. by H.G. Karge, J. Weitkamp (Springer, Berlin, 2008), p. 1
M. Niwa, N. Katada, K. Okumura, Characterization and Design of Zeolite Catalysts (Springer, Berlin, 2010), p. 13
Y. Kobayashi, Y.X. Li, Y. Wang, D.Z. Wang, Chin. J. Catal. 34, 2192 (2013)
F. Haase, J. Sauer, J. Hutter, Chem. Phys. Lett. 266, 397 (1997)
J. Van der Mynsbrugge, K. Hemelsoet, M. Vandichel, M. Waroquier, V. Van Speybroeck, J. Phys. Chem. C 116, 5499 (2012)
A.J. Jones, R.T. Carr, S.I. Zones, E. Iglesia, J. Catal. 312, 58 (2014)
A.J. Jones, S.I. Zones, E. Iglesia, J. Phys. Chem. C 118, 17787 (2014)
C.M. Nguyen, B.A. DeMoor, M.F. Reyniers, G.B. Marin, J. Phys. Chem. C 115, 23831 (2011)
B.A. DeMoor, M.F. Reyniers, O.C. Gobin, J.A. Lercher, G.B. Marin, J. Phys. Chem. C 115, 1204 (2011)
F. Eder, M. Stockenhuber, J.A. Lercher, J. Phys. Chem. B 101, 5414 (1997)
G. Sastre, A. Corma, J. Mol. Catal. A 305, 3 (2009)
R. Krishna, J.M. van Baten, Sep. Purif. Technol. 76, 325 (2010)
N. Heymans, B. Alban, S. Moreau, G. DeWeireld, Chem. Eng. Sci. 66, 3850 (2011)
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21173125).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, F., Kobayashi, Y., Li, Y. et al. The significance of a second adsorption phase with weakly adsorbed species for the calculation of the surface concentrations of a mixture: methanol–DME and methanol–ethene adsorption in SAPO-34. Res Chem Intermed 41, 9561–9573 (2015). https://doi.org/10.1007/s11164-015-1981-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-1981-x