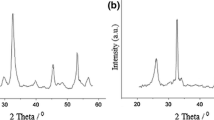
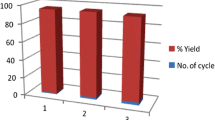
Abstract
Ultrasonic irradiation is being considered not only as a green approach but also as a powerful technique for the synthesis of 1,4-dihydropyridine and imidazo[1,2-a]quinoline derivatives. It can be carried out by using multicomponent reaction of cyclic enaminoketones, malononitrile, and aromatic aldehydes, in the presence of catalytic amounts of zinc oxide nanoparticles, in EtOH, at 80 °C. The preponderance of such a catalyst is due to its inexpensiveness, stability, and the potential of being easily obtained. Furthermore, high conversions, short reaction times, and cleaner reaction profiles are some of the advantages of this method.


Similar content being viewed by others
References
T.J. Mason, J.P. Lorimer, In Sonochemistry: Theory Application and Uses of Ultrasound in Chemistry (Wiley, New York, 1988)
J.L. Luche, Synthetic Organic Sonochemistry (Plenum, New York, 1998)
H.J. Zang, M.L. Wang, B.W. Cheng, J. Song, Ultrason. Sonochem. 16, 301 (2009)
J.T. Li, M.X. Sun, Y. Yin, Ultrason. Sonochem. 17, 359 (2010)
A. Bazgir, S. Ahadi, R. Ghahremanzadeh, H.R. Khavasi, P. Mirzaei, Ultrason. Sonochem. 17, 447 (2010)
A. Dandia, R. Singh, S.L. Gupta, Res. Chem. Intermed. (2013). doi:10.1007/s11164-013-1292-z
E. Chandralekha, A. Thangamani, R. Valliappan, Res. Chem. Intermed. 39, 961 (2013)
A.S. Al-bogami, Res. Chem. Intermed. (2013). doi:10.1007/s11164-013-1171-7
R. Sandaroos, S. Damavandi, Res. Chem. Intermed. 39, 4167 (2013)
R.V.A. Orru, M. de Greef, Synthesis 10, 1471 (2003)
A. Domling, I. Ugi, Angew. Chem. Int. Ed. 39, 3168 (2000)
H. Ohno, Y. Ohta, S. Oishi, N. Fujii, Angew. Chem. Int. Ed. 46, 2295 (2007)
H. Yoshida, H. Fukushima, J. Ohshita, A. Kunai, J. Am. Chem. Soc. 128, 11040 (2006)
H.A. Dondas, C.W.G. Fishwick, X. Gai, R. Grigg, C. Kilner, N. Dumrongchai, B. Kongkathip, N. Kongkathip, C. Polysuk, V. Sridharan, Angew. Chem. Int. Ed. 44, 7570 (2005)
A.R. Siamaki, B.A. Arndtsen, J. Am. Chem. Soc. 128, 6050 (2006)
E.C. Franklin, Chem. Rev. 16, 305 (1935)
F.W. Bergstrom, Chem. Rev. 35, 77 (1944)
D.M. Stout, A. Meyers, Chem. Rev. 82, 223 (1982)
R. Boer, V. Gekeler, Drugs Future 20, 499 (1995)
M. Ramesh, W.V. Matowe, J. Med. Chem. 41, 509 (1998)
S.R. Pattan, A.N. Parate, Indian J. Heterocycl. Chem. 12, 387 (2003)
Y.S. Sadanandam, M.M. Shetty, Eur. J. Med. Chem. 29, 975 (1994)
K. Cooper, M.J. Fray, J. Med. Chem. 35, 3115 (1992)
S.R. Agudoawu, E.E. Knaus, J. Heterocycl. Chem. 37, 303 (2000)
A. Shafiee, N. Rastakari, Daru 12, 81 (2004)
I.R. Ager, A.C. Barnes, G.W. Danswan, P.W. Hairsine, D.P. Kay, P.D. Kennewell, S.S. Matharu, P. Miller, P. Robson, D.A. Rowlands, W.R. Tully, R. Westwood, J. Med. Chem. 31, 1098 (1988)
S.J. Clements, G. Danswan, C.R. Gardner, S.S. Matharu, R. Murdoch, W.R. Tully, R. Westwood, J. Med. Chem. 31, 1220 (1988)
N.R. Shiju, V.V. Guliants, Appl. Catal. A Gen. 356, 1 (2009)
J. Guzman, B.C. Gates, Nano Lett. 1, 689 (2001)
B.M. Choudary, M.L. Kantam, K.V.S. Ranganath, K. Mahender, B. Sreedhar, J. Am. Chem. Soc. 126, 3396 (2004)
S. Anadan, A. Vinu, N. VenkataChalam, B. Arabindoo, V. Murugesan, J. Mol. Catal. A Chem. 256, 312 (2006)
K.J. Klabunde, R. Mulukutla, Nanoscale Materials in Chemistry (Wiley Interscience, New York, 2001)
A.J. Amali, R.K. Rana, Green Chem. 11, 1781 (2009)
R. Schlogl, S.B. Abd, Chem. Int. Ed. 43, 1628 (2003)
A.T. Bell, Science 299, 1688 (2003)
Z. Lasemi, E. Mehrasbi, Res. Chem. Intermed. (2013). doi:10.1007/s11164-013-1394-7
B.M. Choudary, K. Mahendar, K.V.S. Ranganath, J. Mol. Catal. A Chem. 234, 25 (2005)
M.H. Sarvari, H. Sharghi, J. Org. Chem. 69, 2573 (2004)
F. Tamaddon, M.A. Amrollahi, L. Sharafat, Tetrahedron Lett. 46, 7841 (2005)
B.V. Lichitsky, A.A. Dudinov, M.M. Krayushkin, Arkivoc ix, 73 (2012)
S. Tu, C. Li, G. Li, L. Cao, Q. Shao, D. Zhou, B. Jiang, J. Zhou, M. Xia, J. Comb. Chem. 9, 1144 (2007)
D.S. Patel, J.R. Avalani, D.K. Raval, J. Braz. Chem. Soc. 23, 1951 (2012)
Z. Karimi-Jaberi, Z. Takmilifard, Eur. Chem. Bull. 2, 211 (2013)
M. Hosseini-Sarvari, S. Etemad, Tetrahedron 64, 5519 (2008)
Acknowledgments
The authors express their great appreciation to Kerman University of Medical Sciences, Pharmaceutics Research Center, Institute of Neuropharmacology for support of this investigation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abaszadeh, M., Seifi, M. & Asadipour, A. Ultrasound promotes one-pot synthesis of 1,4-dihydropyridine and imidazo[1,2-a]quinoline derivatives, catalyzed by ZnO nanoparticles. Res Chem Intermed 41, 5229–5238 (2015). https://doi.org/10.1007/s11164-014-1624-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1624-7