Abstract
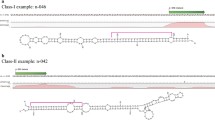
MicroRNA genes (miRNAs) encoding small non-coding RNAs are abundant in plant genomes and play a key role in regulating several biological mechanisms. Five conserved miRNAs, miR156, miR168-1, miR168-2, miR164, and miR166 were selected for analysis from the 21 known plant miRNA families that were recovered from deep sequencing data of small RNA libraries of pumpkin and squash. A total of six novel miRNAs that were not reported before were found to have precursors with reliable fold-back structures and hence considered novel and were designated as cuc_nov_miRNAs. A set of five conserved, six novel miRNAs, and five uncharacterized small RNAs from the deep sequencing data were profiled for their dynamic regulation using qPCR. The miRNAs were evaluated for differential regulation across the tissues among four diverse cucurbit species, including pumpkin and squash (Cucurbita moschata Duch. Ex Poir. and Cucurbita pepo L.), bitter melon (Momordica charantia L.), and Luffa (Loofah) (Luffa acutangula Roxb.). Expression analysis revealed differential regulation of various miRNAs in leaf, stem, and fruit tissues. Importantly, differences in the expression levels were also found in the leaves and fruits of closely related C. moschata and C. pepo. Comparative miRNA profiling and expression analysis in four cucurbits led to identification of conserved miRNAs in cucurbits. Predicted targets for two of the conserved miRNAs suggested miRNAs are involved in regulating similar biological mechanisms in various species of cucurbits.



Similar content being viewed by others
References
Abdel-Ghany S, Pilon M (2008) MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem 283:15932–15945
Bartel D (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bartel B, Bartel DP (2003) MicroRNAs: at the root of plant development? Plant Physiol 132:709–717. doi:10.1104/pp. 103.023630
Borsani O, Zhu J, Verslues P, Sunkar R, Zhu J (2005) Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulates salt tolerance in Arabidopsis. Cell 123:1279–1291
Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto Y, Sieburth L, Voinnet O (2008) Widespread translational inhibition by plant miRNAs and siRNAs. Science 320:1185–1190
Cuperus JT, Fahlgren N, Carrington JC (2011) Evolution and functional diversification of MIRNA genes. Plant Cell 23:431–442. doi:10.1105/tpc.110.082784
Fahlgren N, Howell MD, Kasschau KD, Chapman EJ, Sullivan CM, Cumbie JS, Givan SA, Law TF, Grant SR, Dangl JL, Carrington JC (2007) High-throughput sequencing of Arabidopsis microRNAs: evidence for frequent birth and death of MIRNA genes. PLoS One 2(2):e219. doi:10.1371/journal.pone.0000219
Fujii H, Chiou T-J, Lin S-I, Aung K, Zhu J-K (2005) A miRNA involved in phosphate-starvation response in arabidopsis. Curr Biol 15:2038–2043
Gazzani S, Li M, Maistri S, Scarponi E, Graziola M, Barbaro E, Wunder J, Furini A, Saedler H, Varotto C (2009) Evolution of MIR168 paralogs in Brassicaceae. BMC Evol Biol 9:62
Gonzalez-Ibeas D, Blanca J, Donaire L, Saladie M, Mascarell-Creus A, Cano-Delgado A, Garcia-Mas J, Llave C, Aranda M (2011) Analysis of the melon (Cucumis melo) small RNAome by high-throughput pyrosequencing. BMC Genom 12:393
Hammond SM, Bernstein E, Beach D, Hannon GJ (2000) An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404:293–296
Hewezi T, Howe P, Maier TR, Baum TJ (2008) Arabidopsis small RNAs and their targets during cyst nematode parasitism. Mol Plant Microbe Interact 21:1622–1634
Jagadeeswaran G, Nimmakayala P, Zheng Y, Gowdu K, Reddy UK, Sunkar R (2012) Characterization of the small RNA component of leaves and fruits from four different cucurbit species. BMC Genom 13:329
Jones-Rhoades MW, Bartel DP (2004) Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol cell 14:787–799. doi:10.1016/j.molcel.2004.05.027
Jung J-H, Park C-M (2007) 165 genes exhibit dynamic expression patterns in regulating shoot apical meristem and floral development in Arabidopsis. Planta 225:1327–1338. doi:10.1007/s00425-006-0439-1
Katiyar-Agarwal S, Gao S, Vivian-Smith A, Jin H (2007) A novel class of bacteria-induced small RNAs in Arabidopsis. Genes Dev 21:1–12. doi:10.1101/gad.1595107
Kim V (2005) MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol 6:376–385
Kurihara Y, Watanabe Y (2004) Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc Natl Acad Sci USA 101:12753–12758
Laufs P, Peaucelle A, Morin H, Traas J (2004) MicroRNA regulation of the CUC genes is required for boundary size control in Arabidopsis meristems. Development 131:4311–4322. doi:10.1242/dev.01320
Liu W-M, Pang RTK, Cheong AWY, Ng EHY, Lao K et al (2012) Involvement of microRNA Lethal-7a in the regulation of embryo implantation in mice. PLoS One 7:e37039. doi:10.1371/journal.pone.0037039
Lu C, Fedoroff N (2000) A mutation in the arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell 12:2351–2366. doi:10.1105/tpc.12.12.2351
Mallory AC, Reinhart BJ, Jones-Rhoades MW, Tang G, Zamore PD, Barton MK, Bartel DP (2004) MicroRNA control of PHABULOSA in leaf development: importance of pairing to the microRNA 5[prime] region. EMBO J 23:3356–3364
Martin RC, Liu P-P, Goloviznina NA, Nonogaki H (2010) microRNA, seeds, and Darwin?: diverse function of miRNA in seed biology and plant responses to stress. J Exp Bot 61:2229–2234. doi:10.1093/jxb/erq063
Martínez G, Forment J, Llave C, Pallás V, Gómez G (2011) High-throughput sequencing, characterization and detection of new and conserved cucumber miRNAs. PLoS One 6:19523. doi:10.1371/journal.pone.0019523
Mohorianu I, Schwach F, Jing R, Lopez-Gomollon S, Moxon S, Szittya G, Sorefan K, Moulton V, Dalmay T (2011) Profiling of short RNAs during fleshy fruit development reveals stage-specific sRNAome expression patterns. Plant J 67:232–246
Moxon S, Jing R, Szittya G, Schwach F, Rusholme Pilcher RL, Moulton V, Dalmay T (2008) Deep sequencing of tomato short RNAs identifies microRNAs targeting genes involved in fruit ripening. Genome Res 18:1602–1609. doi:10.1101/gr.080127.108
Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones J (2006) A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312:436–439
Pandey SP, Shahi P, Gase K, Baldwin IT (2008) Herbivory-induced changes in the small-RNA transcriptome and phytohormone signaling in Nicotiana attenuate. Proc Natl Acad Sci U S A 105:4559–4564
Perez-Llamas C, Lopez-Bigas N (2011) Gitools: analysis and visualisation of genomic data using interactive heat-maps. PLoS One 6:e19541. doi:10.1371/journal.pone.0019541
Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP (2002) MicroRNAs in plants. Genes Dev 16:1616–1626. doi:10.1101/gad.1004402
Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP (2002) Prediction of plant MicroRNA targets. Cell 110:513–520. doi:10.1016/s0092-8674(02)00863-2
Sattar S, Song Y, Anstead JA, Sunkar R, Thompson GA (2012) Cucumis melo microRNA expression profile during aphid herbivory in a resistant and susceptible interaction. Mol Plant Microbe Interact 25:839–848
Schaefer H, Heibl C, Renner SS (2009) Gourds afloat: a dated phylogeny reveals an Asian origin of the gourd family (Cucurbitaceae) and numerous oversea dispersal events. Proc Roy Soc B Biol Sci 276:843–851. doi:10.1098/rspb.2008.1447
Schwab R, Palatnik JF, Riester M, Schommer C, Schmid M, Weigel D (2005) Specific effects of microRNAs on the plant transcriptome. Dev cell 8:517–527
Sieber P, Wellmer F, Gheyselinck J, Riechmann JL, Meyerowitz EM (2007) Redundancy and specialization among plant microRNAs: role of the MIR164 family in developmental robustness. Development 134:1051–1060. doi:10.1242/dev.02817
Subramanian S, Fu Y, Sunkar R, Barbazuk WB, Zhu J-K, Yu O (2008) Novel and nodulation-regulated microRNAs in soybean roots. BMC Genom 9:160
Sunkar R (2010) MicroRNAs with macro-effects on plant stress responses. Semin Cell Dev Biol 21:805–811. doi:10.1016/j.semcdb.2010.04.001
Sunkar R, Zhu J-K (2004) Novel and stress-regulated MicroRNAs and other small RNAs from Arabidopsis. Plant Cell 16:2001–2019. doi:10.1105/tpc.104.022830
Sunkar R, Kapoor A, Zhu JK (2006) Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 18:2051–2065. doi:10.1105/tpc.106.041673
Tang G, Reinhart BJ, Bartel DP, Zamore PD (2003) A biochemical framework for RNA silencing in plants. Genes Dev 17:49–63. doi:10.1101/gad.1048103
Varkonyi-Gasic E, Wu R, Wood M, Walton E, Hellens R (2007) Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Meth 3:12
Voinnet O (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136:669–687
Wang F, Liu R, Wu G, Lang C, Chen J, Shi C (2012) Specific downregulation of the bacterial-type PEPC gene by artificial microRNA improves salt tolerance in Arabidopsis. Plant Mol Biol Rep. doi:10.1007/s11105-012-0418-6
Wu G, Park M, Conway S, Wang J, Weigel D, Poethig R (2009) The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138:750–759
Xie Z, Kasschau KD, Carrington JC (2003) Negative feedback regulation of Dicer-like1 in Arabidopsis by microRNA-guided mRNA degradation. Curr Biol 13:784–789. doi:10.1016/s0960-9822(03)00281-1
Xie Z, Allen E, Fahlgren N, Calamar A, Givan SA, Carrington JC (2005) Expression of Arabidopsis MIRNA genes. Plant Physiol 138:2145–2154. doi:10.1104/pp. 105.062943
Xue L-J, Zhang J-J, Xue H-W (2009) Characterization and expression profiles of miRNAs in rice seeds. Nucleic Acids Res 37:916–930. doi:10.1093/nar/gkn998
Yang Y, Chen X, Chen J, Xu H, Li J, Zhang Z (2011) Identification of novel and conserved microRNAs in Rehmannia glutinosa L. by Solexa sequencing. Plant Mol Biol Rep 29:986–996
Zhou J, Zhuo R, Liu M, Qiao G, Jiang J, Li H, Qiu W, Zhang X, Lin S (2011) Identification and characterization of novel microRNAs from Populus cathayana. Rehd Plant Mol Biol Rep 29:242–2511
Acknowledgments
The authors are grateful to Dr. Jarret, Plant Genetic Resources Conservation Unit, USDA-ARS, Griffin, GA, 30223 for providing the seeds of germplasm accessions. Funding support is provided by NSF-EPSCOR no. 1003907, Gus R. Douglass Institute, and USDA-NIFA Research (2010-38821-21476).
Author information
Authors and Affiliations
Corresponding author
Additional information
Umesh K. Reddy contributed equally with the first author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Heatmap of expression profiles of conserved miRNAs in leaf, stem, flesh, rind, and placenta of various cucurbit species. The color white to light blue to dark blue to black represents the gradation of scale of relative miRNA expression (log2). (JPEG 44 kb)
Supplementary Fig. 2
Heatmap of expression profiles of novel miRNAs in leaf, stem, flesh, rind, and placenta of various cucurbit species. The color white to light blue to dark blue to black represents the gradation of scale of relative miRNA expression (log2). (JPEG 46 kb)
Supplementary Fig. 3
Heatmap of expression profiles of small RNAs in leaf, stem, flesh, rind, and placenta of various cucurbit species. The color white to light blue to dark blue to black represents the gradation of scale of relative miRNA expression (log2). (JPEG 43 kb)
Rights and permissions
About this article
Cite this article
Manohar, S., Jagadeeswaran, G., Nimmakayala, P. et al. Dynamic Regulation of Novel and Conserved miRNAs Across Various Tissues of Diverse Cucurbit Species. Plant Mol Biol Rep 31, 335–343 (2013). https://doi.org/10.1007/s11105-012-0506-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11105-012-0506-7