Abstract
Aims
The present study was undertaken to investigate the mechanism underlying NaCl-alleviated Fe (iron) deficiency in Arabidopsis thaliana Col-0 (Columbia ecotype).
Methods
Six-week-old Col-0 with rosettes of similar diameters was grown in full-strength nutrient solution lacking Fe (Fe-deficient) or full-strength nutrient solution (Fe-sufficient) with or without 10 mM NaCl for 7 days. Roots were then harvested for analysis of total Fe content, soluble Fe concentration, and abscisic acid (ABA) content as well as for cell wall and RNA extraction, while shoots were harvested for total Fe content and soluble Fe concentration measurements.
Results
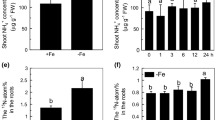
The total Fe content and the soluble Fe concentration were higher in roots and shoots of −Fe + NaCl-treated plants than −Fe-treated plants, whereas Fe retention in the cell wall was reduced, suggesting the presence of a cell wall Fe-reutilization mechanism. This conclusion was confirmed by the observation that less hemicelluloses Fe was found in plants under −Fe + NaCl treatment. In addition, associated to the upregulation of genes related to the long-distance transport of Fe, such as FRD3 (FERRIC REDUCTASE DEFECTIVE3), YSL2 (YELLOW STRIPE-LIKE), and NAS1 (NICOTIANAMINE SYNTHASE1) under −Fe + NaCl treatment, more Fe was available in shoots. Furthermore, endogenous ABA is involved in NaCl-alleviated Fe deficiency, as the addition of Flu (fluridone), an inhibitor of ABA biosynthesis, abolished the positive effect of NaCl on Fe deficiency.
Conclusions
Under −Fe condition, NaCl not only is involved in the reutilization of cell wall Fe but also participates in the translocation of Fe from root to shoot in Arabidopsis, partially through its effect on ABA contents.









Similar content being viewed by others
References
Ali L, Rahmatullah, Ranjha AM, Aziz T, Maqsood MA, Ashraf M (2006) Differential potassium requirement and its substitution by sodium in cotton genotypes. Pak J Agric Sci 43:3–4
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285:1256–1258
Anderegg G, Ripperger H (1989) Correlation between metal complex formation and biological activity of nicotianamine analogues. J Chem Soc Chem Commun 10:647–650
Battie-Laclau P, Laclau J-P, Piccolo MC, Arenque BC, Beri C, Mietton L, Muniz MRA, Jordan-Meille L, Buckeridge MS, Nouvellon Y, Ranger J, Bouillet JP (2013) Influence of potassium and sodium nutrition on leaf area components in Eucalyptus grandis trees. Plant Soil 371:19–35
Bernstein N, Shoresh M, Xu Y, Huang B (2010) Involvement of the plant antioxidative response in the differential growth sensitivity to salinity of leaves vs roots during cell development. Free Radical Bio Med 49:1161–1171
Box S, Schachtman DP (2000) The effect of low concentrations of sodium on potassium uptake and growth of wheat. Funct Plant Biol 27:175–182
Brown JC, Ambler JE (1973) “Reductants” released by roots of Fe-deficient soybeans. Agron J 65:311–314
Brownell PF, Crossland CJ (1972) The requirement for sodium as a micronutrient by species having the C4 dicarboxylic photosynthetic pathway. Plant Physiol 49:794–797
Conde A, Silva P, Agasse A, Conde C, Geros H (2011) Mannitol transport and mannitol dehydrogenase activities are coordinated in Olea europaea under salt and osmotic stresses. Plant Cell Physiol 52:1766–1775
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Bio 6:850–861
Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54:183–206
Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL (2001) Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409:346–349
Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inze D, Van Breusegen F (2000) Dual action of the active oxygen species during plant stress responses. CMLS 57:779–995
DeCosta W, Zörb C, Hartung W, Schubert S (2007) Salt resistance is determined by osmotic adjustment and abscisic acid in newly developed maize hybrids in the first phase of salt stress. Physiol Plant 131:311–321
DiDonato RJ, Roberts LA, Sanderson T, Eisley RB, Walker EL (2004) Arabidopsis Yellow Stripe-Like2 (YSL2): a metal-regulated gene encoding a plasma membrane transporter of nicotianamine-metal complexes. Plant J 39:403–414
Ding Z, Li S, An X, Liu X, Qin H, Wang D (2009) Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana. J Genet Genomics 36:17–29
Durrett TP, Gassmann W, Rogers EE (2007) The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol 144:197–205
Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci U S A 93:5624–5628
Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14:S15–S45
Gattward JN, Almeida AAF, Souza JO, Gomes FP, Kronzucker HJ (2012) Sodium-potassium synergism in Theobroma cacao: stimulation of photosynthesis, water-use efficiency and mineral nutrition. Physiol Plant 146:350–362
Green LS, Rogers EE (2004) FRD3 controls iron localization in Arabidopsis thaliana. Plant Physiol 136:2523–2531
Guerinot ML, Yi Y (1994) Iron: nutritious, noxious, and not readily available. Plant Physiol 104:815–820
Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ (2000) Plant cellular and molecular responses to high salinity. Annu Rev Plant Biol 51:463–499
Haydon MJ, Cobbett CS (2007) Transporters of ligands for essential metal ions in plants. New Phytol 174:499–506
He JY, Zhu C, Ren YF, Yan YP, Cheng C, Jiang DA, Sun ZX (2008) Uptake, subcellular distribution, and chemical forms of cadmium in wild-type and mutant rice. Pedosphere 18:371–377
Higuchi K, Suzuki K, Nakanishi H, Yamaguchi H, Nishizawa NK, Mori S (1999) Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol 119:471–479
Jin CW, You GY, He YF, Tang CX, Wu P, Zheng SJ (2007) Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol 144:278–285
Kim SA, Punshon T, Lanzirotti A, Li LT, Alonso JM, Ecker JR, Guerinot L (2006) Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314:1295–1298
Klatte M, Schuler M, Wirtz M, Straube CF, Hell R, Bauer P (2009) The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses. Plant Physiol 150:257–271
Kravchik M, Bernstein N (2013) Effects of salinity on the transcriptome of growing maize leaf cells point at cell-age specificity in the involvement of the antioxidative response in cell growth restriction. BMC Genomics 14:1
Lanquar V, Lelièvre F, Bolte S, Hamès C, Alcon C, Neumann D, Thomine S (2005) Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J 24:4041–4051
Lei GJ, Zhu XF, Wang ZW, Dong F, Dong NY, Zheng SJ (2014) Abscisic acid alleviates iron deficiency by promoting root iron reutilization and transport from root to shoot in Arabidopsis. Plant Cell Environ 37:852–863
Li BH, Li Q, Xiong LM, Kronzucker HJ, Kramer U, Shi WM (2012) Arabidopsis plastid AMOS1/EGY1 integrates abscisic acid signaling to regulate global gene expression response to ammonium stress. Plant Physiol 160:2040–2051
Ma JF, Zheng SJ, Hiradate S, Matsumoto H (1997) Detoxifying aluminum with buckwheat. Nature 390:569–570
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Munns R, Tester M (2008) Mechanism of salinity tolerance. Annu Rev Plant Biol 59:651–681
Murgia I, Vazzola V, Tarantino D, Cellier F, Ravet K, Briat JF, Sovae C (2007) Knock-out of ferritin AtFer1 causes earlier onset of age-dependent leaf senescence in Arabidopsis. Plant Physiol Biochem 45:898–907
Nambara E, Marion-Poll A (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56:165–185
Neves-Piestun BG, Bernstein N (2005) Salinity-induced changes in the nutritional status of expanding cells may impact leaf growth inhibition in maize. Funct Plant Biol 32:141–152
Rengasamy P (2006) World salinization with emphasis on Australia. J Exp Bot 57:1017–1023
Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric chelate reductase for iron uptake from soils. Nature 397:694–697
Rogers EE, Guerinot ML (2002) FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell 14:1787–1799
Roschzttardtz H, Séguéla-Arnaud M, Briat JF, Vert G, Curie C (2011) The FRD3 citrate effluxer promotes iron nutrition between symplastically disconnected tissues throughout Arabidopsis development. Plant Cell 23:2725–2737
Römheld V, Marschner H (1981) Iron deficiency stress induced morphological and physiological changes in root tips of sunflower. Plant Physiol 53:354–360
Santi S, Schmidt W (2009) Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol 183:1072–1084
Schmohl N, Horst WJ (2000) Cell wall pectin content modulates aluminium sensitivity of Zea mays (L.) cells grown in suspension culture. Plant Cell Environ 23:735–742
Seo M, Peeters AJ, Koiwai H, Oritani T, Marion-Poll A, Zeevaart JA, Koornneef M, Kamiya Y, Koshiba T (2000) The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc Natl Academy Sci USA 97:12908–12913
Singh NK, Larosa PC, Handa AK, Hasegawa PM, Bressan RA (1987) Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc Natl Acad Sci U S A 84:739–743
Singleton VL, Rossi JAJ (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Viticult 16:144–158
Sivakumar P, Sharmila P, Saradhi PP (2000) Proline alleviates salt-stress-induced enhancement in ribulose-1, 5-bisphosphate oxygenase activity. Biochem Biophys Res Commun 279:512–515
Taiz L, Zeiger E (2010) Plant physiol. Sinauer Associates, Sunderland, MA
Thomine S, Lelièvre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H (2003) AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J 34:685–695
Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14:1223–1233
Waters BM, Chu HH, DiDonato RJ, Roberts LA, Eisley RB, Lahner B, Walker EL (2006) Mutations in Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol 141:1446–1458
Woolley JT (1957) Sodium and silicon as nutrients for the tomato plant. Plant Physiol 32:317
Xiong L, Ishitani M, Lee H, Zhu JK (2001) The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 13:2063–2083
Xiong L, Zhu JK (2003) Regulation of abscisic acid biosynthesis. Plant Physiol 133:29–36
Yang JL, Chen WW, Chen LQ, Qin C, Jin CW, Shi YZ, Zheng SJ (2013) The 14-3-3 protein GENERAL REGULATORY FACTOR11 (GRF11) acts downstream of nitric oxide to regulate iron acquisition in Arabidopsis thaliana. New Phytol 197:815–824
Yokosho K, Yamaji N, Ueno D, Mitani N, Ma JF (2009) OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol 149:297–305
Yoshino M, Murakami K (1998) Interaction of iron with polyphenolic compounds: application to antioxidant characterization. Anal Biochem 257:40–44
Yoshioka T, Endo T, Satoh S (1998) Restoration of seed germination at supraoptimal temperatures by fluridone, an inhibitor of abscisic acid biosynthesis. Plant Cell Physiol 39:307–312
Zhu C, Schraut D, Hartung W, Schäffner AR (2005) Differential responses of maize MIP genes to salt stress and ABA. J Exp Bot 56:2971–2981
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Zhu XF, Shi YZ, Lei GJ, Fry SC, Zhang BC, Zhou YH, Zheng SJ (2012a) XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell 24:4731–4747
Zhu XF, Lei GJ, Jiang T, Liu Y, Li GX, Zheng SJ (2012b) Cell wall polysaccharides are involved in P-deficiency-induced Cd exclusion in Arabidopsis thaliana. Planta 236:989–997
Zhu XF, Wang ZW, Wan JX, Sun Y, Wu YR, Li GX, Shen RF, Zheng SJ (2015) Pectin enhances rice (Oryza sativa) root phosphorus remobilization. J Exp Bot 66:1017–1024
Zhu XF, Wang B, Song WF, Zheng SJ, Shen RF (2016) Putrescine alleviates iron deficiency via NO-dependent reutilization of root cell-wall Fe in Arabidopsis. Plant Physiol 170:558–567
Zörb C, Geilfus CM, Mühling KH, Ludwig-Müller J (2013) The influence of salt stress on ABA and auxin concentrations in two maize cultivars differing in salt resistance. J Plant Physiol 170:220–224
Acknowledgements
This work was funded by the National Key Basic Research Program of China (No. 2014CB441000) and “Strategic Priority Research Program” of the Chinese Academy of Sciences (Nos. XDB15030302 and XDB15030202). We thank three anonymous reviewers for their valuable comments to improve the quality of our work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Guillermo Santa Maria.
Rights and permissions
About this article
Cite this article
Zhu, X.F., Wu, Q., Zheng, L. et al. NaCl alleviates iron deficiency through facilitating root cell wall iron reutilization and its translocation to the shoot in Arabidopsis thaliana . Plant Soil 417, 155–167 (2017). https://doi.org/10.1007/s11104-017-3248-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3248-3