Abstract
Background and aims
Take-all, caused by the soilborne pathogen Gaeumannomyces graminis var. tritici, (Ggt), is an important root disease of wheat. Continuous wheat cropping has been shown to induce take-all decline (TAD). This research investigated the mechanisms of TAD in 13 New Zealand soils in two experiments and identified the associated microorganisms using denaturing gradient gel electrophoresis (DGGE).
Methods
In Experiment 1, a sterile sand/maize-meal mixture inoculated or not inoculated with Ggt, was added at 4 % (w/w) to sterilised and non-sterilised soils to determine their ability to suppress take-all, and to help identify the nature of suppression. Experiment 2 investigated the transferability of suppressive properties in five of the soils from Experiment 1. The microbial communities of these five soils were analysed using PCR-DGGE.
Results
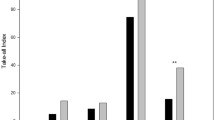
Ten of the soils were able to suppress take-all but the suppression was biological in nature in only four of these soils. The suppressive properties of two of the soils were transferred to a γ-irradiated base soil amended with Ggt, indicating that suppression could be specific in nature (i.e., attributed to a specific microorganism or group of microorganisms). The suppressive properties in one soil were not transferrable, suggesting a general form of suppression, most probably because the conditions in the soil were suitable for other microorganisms to compete with Ggt. DGGE analyses of the microbial communities for five of the soils showed similar banding patterns for those with similar forms of suppression (specific, general and non-suppressive) and identified the potential microorganisms that distinguished them.
Conclusion
These distinguishing microorganisms are likely to independently or interactively have a function in suppressing take-all.






Similar content being viewed by others
References
Aberra MB, Seah S, Sivasithamparam K (1998) Suppression of the take-all fungus (Gaeumannomyces graminis var. tritici) by a sterile red fungus through induced resistance in wheat (Triticum aestivum) seedling roots. Soil Biol Biochem 30:1457–1461
Alef K (1995) Sterilisation of soil and inhibition of microbial activity. In: Nannipieri AKP (ed) Methods in applied soil microbiology and biochemistry. Academic, London, pp 52–54
Andjic V, Cole ALJ, Klena JD (2005) Taxonomic identity of the sterile red fungus inferred using nuclear rDNA ITS 1 sequences. Mycol Res 109:200–204
Andrade OA, Mathre DE, Sands DC (1994a) Natural suppression of take-all disease of wheat in Montana soils. Plant Soil 164:9–18
Andrade OA, Mathre DE, Sands DC (1994b) Suppression of Gaeumannomyces graminis var. tritici in Montana soils and its transferability between soils. Soil Biol Biochem 26:397–402
Augustin C, Jacob HJ, Werner A (1997) Effects on growth of wheat plants of isolates of Gaeumannomyces/Phialophora-complex fungi in different conditions of soil moisture, temperature, and photoperiod. Eur J Plant Pathol 103:417–426
Baker KF, Cook RJ (1974) Biological control of plant pathogens. Freeman, San Francisco
Bithell SL, McKay A, Butler RC, Ophel-Keller K, Hartley D, Cromey MG (2012) Predicting take-all severity in second-year wheat using soil DNA concentrations of Gaeumannomyces graminis var. tritici determined with qPCR. Plant Dis 96:443–451
Brisbane PG, Rovira AD (1988) Mechanisms of inhibition of Gaeumannomyces graminis var. tritici by fluorescent pseudomonads. Plant Pathol 37:104–111
Chng SF, Cromey MG, Butler RC (2005) Evaluation of the susceptibility of various grass species to Gaeumannomyces graminis var. tritici. New Zealand Plant Protection, Vol 58. Proceedings of a conference Wellington, New Zealand, pp 261–267
Chng SF, Stewart A, Cromey MG, Dodd SL, Butler RC, Jaspers MV (2013) Effects of different rates of Gaeumannomyces graminis var. tritici inoculum for detecting take-all suppression in soils. Australas Plant Pathol 42:103–109
Christen D, Tharin M, Perrin-Cherioux S, Abou-Mansour E, Tabacchi R, Defago G (2005) Transformation of Eutypa dieback and Esca disease pathogen toxins by antagonistic fungal strains reveals a second detoxification pathway not present in Vitis vinifera. J Agric Food Chem 53:7043–7051
Conn VM (2005) Molecular interactions of endophytic actinobacteria in wheat and arabidopsis. PhD thesis. Flinder University
Conn VM, Franco CMM (2004) Effect of microbial inoculants on the indigenous actinobacterial endophyte population in the roots of wheat as determined by terminal restriction fragment length polymorphism. Appl Environ Microbiol 70:6407–6413
Cook RJ (2003) Take-all of wheat. Physiol Mol Plant Pathol 62:73–86
Cook RJ, Rovira AD (1976) The role of bacteria in the biological control of Gaeumannomyces graminis by suppressive soils. Soil Biol Biochem 8:269–273
Cook RJ, Wilkinson HT, Alldredge JR (1986) Evidence that microorganisms in suppressive soil associated with wheat take-all decline do not limit the number of lesions produced by Gaeumannomyces graminis var. tritici. Phytopathology 76:342–345
Coombs JT, Franco CMM (2003) Isolation and identification of actinobacteria from surface-sterilized wheat roots. Appl Environ Microbiol 69:5603–5608
Coombs JT, Michelsen PP, Franco CMM (2004) Evaluation of endophytic actinobacteria as antagonists of Gaeumannomyces graminis var. tritici in wheat. Biol Control 29:359–366
Cromey MG, Fraser PM, Francis GS, Mckay A, Stewart A, Walter M, Waipara NW, Wakelin S (2004) nfluence of crop management on the incidence of take-all of cereals in New Zealand. Proceedings of the third Australasian soilborne diseases symposium, Adelaide, pp 61–62
Deacon JW (1976) Biological control of the take-all fungus, Gaeumannomyces graminis, by Phialophora radicicola and similar fungi. Soil Biol Biochem 8:275–283
Deacon JW (1997) Modern mycology. Blackwell Science, Scotland
Dewan MM, Sivasithamparam K (1988) Occurrence of species of Aspergillus and Penicillium in roots of wheat and ryegrass and their effects on root rot caused by Gaeumannomyces graminis var. tritici. Aust J Bot 36:701–710
Duffy BK, Simon A, Weller DM (1996) Combination of Trichoderma koningii with fluorescent Pseudomonas for control of take-all on wheat. Phytopathology 86:188–194
Dyke GV, Slope DB (1978) Effects of previous legume and oat crops on grain yield and take-all in spring barley. J Agric Sci UK 91:443–451
Garbeva P, van Veen JA, van Elsas JD (2004) Microbial diversity in soil: selection of microbial populations by plant and soil type and implications for disease suppressiveness. Annu Rev Phytopathol 42:243–270
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Garrett SD (1970) Pathogenic root-infecting fungi. Cambridge University Press, London, p 294
Garrett SD (1975) Cellulolysis rate and competitive saprophytic colonisation of wheat straw by foot-rot fungi. Soil Biol Biochem 7:323–327
Garrett SD (1981) Introduction. In: Asher MJC, Shipton PJ (eds) Biology and control of take-all. Academic Press Inc. (London) Ltd, London, pp 1–11
Gerlagh M (1968) Introduction of Ophiobolus graminis into new polders and its decline. Neth J Plant Pathol 74:1–97
Gilligan CA, Brassett PR, Campbell A (1994) Modeling of early infection of cereal roots by the take-all fungus - a detailed mechanistic simulator. New Phytol 128:515–537
Graham JH, Mitchell DJ (1999) Biological control of soilborne plant pathogens and nematodes. In: Sylvia DM, Fuhrmann JJ, Hartel PG, Zuberer DA (eds) Principles and applications of soil microbiology. Prentice Hall, New Jersey, pp 427–446
Harrison LA, Letendre L, Kovacevich P, Pierson E, Weller D (1993) Purification of an antibiotic effective against Gaeumannomyces graminis var. tritici produced by a biocontrol agent, Pseudomonas aureofaciens. Soil Biol Biochem 25:215–221
Heuer H, Smalla K (1997) Application of denaturing gradient gel electrophoresis and temperature gradient gel electrophoresis for studying soil microbial communities. In: Elsas JDV, Trevors JT, Wellington EMH (eds) Modern soil microbiology. Marcel Dekker, Inc, New York, pp 353–373
Heuer H, Krsek M, Baker P, Smalla K, Wellington EMH (1997) Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol 63:3233–3241
Heuer H, Wieland G, Schonfeld J, Schonwalder A, Gomes NCM, Smalla K (2001) Bacterial community profiling using DGGE or TGGE analysis. Environmental Molecular Microbiology : Protocols and Applications. Horizon Scientific Press, Wymondham, pp 177–190
Hiddink GA, Termorshuizen AJ, Raaijmakers JM, Bruggen AHCV (2005) Effect of mixed and single crops on disease suppressiveness of soils. Phytopathology 95:1325–1332
Ho MA, Squire LM, Sabeh NC, Giles DK, VanderGheynst JS (2005) Design and evaluation of a grapevine pruner for biofungicide application. Bioresour Technol 96:963–968
Hollins TW, Scott PR, Gregory RS (1986) The relative resistance of wheat, rye and triticale to take-all caused by Gaeumannomyces graminis. Plant Pathol 35:93–100
Hornby D (1983) Suppressive soils. Annu Rev Phytopathol 21:65–85
Hornby D, Bateman GL, Gutteridge RJ, Ward E, Yarham DJ (1998) Take-all disease of cereals: a regional perspective. CAB International, UK
Janvier C, Villeneuve F, Alabouvette C, Edel-Hermann V, Mateille T, Steinberg C (2007) Soil health through soil disease suppression: which strategy from descriptors to indicators? Soil Biol Biochem 39:1–23
John S, Wicks TJ, Hunt JS, Lorimer MF, Oakey H, Scott ES (2005) Protection of grapevine pruning wounds from infection by Eutypa lata using Trichoderma harzianum and Fusarium lateritium. Australas Plant Pathol 34:569–575
Kim D, Cook RJ, Weller DM (1997) Bacillus sp. L324-92 for biological control of three root diseases of wheat grown with reduced tillage. Phytopathology 87:551–558
Kirk JJ, Deacon JW (1987a) Control of the take-all fungus by Microdochium bolleyi, and interactions involving M. bolleyi, Phialophora graminicola and Periconia macrospinosa on cereal roots. Plant Soil 98:231–237
Kirk JJ, Deacon JW (1987b) Invasion of naturally senescing root cortices of cereal and grass seedlings by Microdochium bolleyi. Plant Soil 98:239–246
Kisand V, Wikner J (2003) Limited resolution of 16S rDNA DGGE caused by melting properties and closely related DNA sequences. J Microbiol Methods 54:183–191
Kloepper JW, Leong J, Teintze M, Schroth M (1980) Pseudomonas siderophores: a mechanism explaining disease-suppressive soils. Curr Microbiol 4:317–320
Kowalchuk GA, Os GJ, Aartrijk J, Veen JA (2003) Microbial community responses to disease management soil treatments used in flower bulb cultivation. Biol Fertil Soils 37:55–63
Kwak Y-S, Weller DM (2013) Take-all of wheat and natural disease suppression: a review. Plant Pathol J 29:125–135. doi:10.5423/PPJ.SI.07.2012.0112
Lascaris D, Deacon JW (1991) Colonization of wheat roots from seed-applied spores of Idriella (Microdochium) bolleyi: a biocontrol agent of take-all. Biocontrol Sci Tech 1:229–240
Lebreton L, Lucas P, Dugas F, Guillerm AY, Schoeny A, Sarniguet A (2004) Changes in population structure of the soilborne fungus Gaeumannomyces graminis var. tritici during continuous wheat cropping. Environ Microbiol 6:1174–1185
Lee YB, Lorenz N, Dick LK, Dick RP (2007) Cold storage and pretreatment incubation effects on soil microbial properties. Soil Sci Soc Am J 71:1299–1305. doi:10.2136/sssaj2006.0245
Manly BFJ (2005) Multivariate statistical methods : a primer, 3rd edn. Chapman and Hall, London
Mazzola M (2002) Mechanisms of natural soil suppressiveness to soilborne diseases. Proceedings of the 9th International Symposium on Microbial Ecology, August 2001, Amsterdam, pp 557–564
Morton J, Craighead M, Stevenson K (2000) Managing soil fertility on cropping farms. New Zealand Fertiliser Manufacturers Research Association and New Zealand Pastoral Agriculture Research Institute Ltd, New Zealand, p 46
Nikolcheva LG, Bärlocher F (2004) Taxon-specific fungal primers reveal unexpectedly high diversity during leaf decomposition in a stream. Mycol Prog 3:41–49
Nübel U, Engelen B, Felske A, Snaidr J, Wieshuber A, Amann RI, Ludwig W, Backhaus H (1996) Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol 178:5636–5643
Ophel-Keller K, McKay A, Hartley D, Herdina CJ (2008) Development of a routine DNA-based testing service for soilborne diseases in Australia. Australas Plant Pathol 37:243–253
Pope AMS (1972) The decline phenomenon in take-all disease of wheat. PhD thesis. University of Surrey
Raaijmakers JM, Weller DM (1998) Natural plant protection by 2,4-diacetylphloroglucinol - producing Pseudomonas spp. in take-all decline soils. Mol Plant-Microbe Interact 11:144–152
Raaijmakers JM, Weller DM (2001) Exploiting genotypic diversity of 2,4-diacetylphloroglucinol-producing Pseudomonas spp.: characterization of superior root-colonizing P. fluorescens strain Q8r1-96. Appl Environ Microbiol 67:2545–2554
Riley IT, Wiebkin S, Hartley D, McKay AC (2010) Quantification of roots and seeds in soil with real-time PCR. Plant Soil 331:151–163. doi:10.1007/s11104-009-0241-5
Rovira AD, Cook RJ (1981) The nature and mechanisms of suppression. In: Asher MJC, Shipton PJ (eds) Biology and control of take-all. Academic, London, pp 385–415
Rovira AD, Wildermuth GB (1981) The nature and mechanisms of suppression. In: Asher MJC, Shipton PJ (eds) Biology and control of take-all. Academic Press Inc. (London) Ltd, London, pp 385–415
Russell J, Bulman S (2005) The liverwort Marchantia foliacea forms a specialized symbiosis with arbuscular mycorrhizal fungi in the genus Glomus. New Phytol 165:567–579
Shankar M, Kurtboke DI, Gillespie-Sasse LMJ, Rowland CY, Sivasithamparam K (1994) Possible roles of competition for thiamine, production of inhibitory compounds, and hyphal interactions in suppression of the take-all fungus by a sterile red fungus. Can J Microbiol 40:478–483
Shipton PJ (1977) Monoculture and soilborne plant pathogens. Annu Rev Phytopathol 15:387–407
Shipton PJ (1981) Saprophytic survival between susceptible crops. In: Asher MJC, Shipton PJ (eds) Biology and control of take-all. Academic Press Inc. (London) Ltd, London, pp 295–316
Shipton PJ, Cook RJ, Sitton JW (1973) Occurrence and transfer of a biological factor in soil that suppresses take-all of wheat in Eastern Washington. Phytopathology 63:511–517
Simon A (1989) Biological control of take-all of wheat by Trichoderma koningii under controlled environmental conditions. Soil Biol Biochem 21:323–326
Sitepu D, Wallace HR (1984) Biological-control of Sclerotinia sclerotiorum in luttuce by Fusarium lateritium. Aust J Exp Agric 24:272–276
Sivasithamparam K, Parker CA (1978) Effects of certain isolates of bacteria and actinomycetes on Gaeumannomyces graminis var. tritici and take-all of wheat. Aust J Bot 26:773–782
Sivasithamparam K, Parker CA (1980) Interaction of certain isolates of soil fungi with Gaeumannomyces graminis var. tritici on agar media. Aust J Bot 28:411–419
Skou JP (1981) Morphology and cytology of the infection process. In: Asher MJC, Shipton PJ (eds) Biology and control of take-all. Academic, London, pp 175–197
Thomashow LS, Weller DM (1988) Role of Phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol 170:3499–3508
Vainio EJ, Hantula J (2000) Direct analysis of wood-inhabiting fungi using denaturing gradient gel electrophoresis of amplified ribosomal DNA. Mycol Res 104:927–936
van Elsas JD, Smalla K, Lilley AK, Bailey MJ (2002) Methods for sampling soil microbes. In: Hurst CJ, Crawford RL, Knudsen GR, McLnerney MJ, Stetzenbach LD (eds) Manual of environmental microbiology, 2nd edn. ASM Press, USA, pp 505–515
van Toor RF, Chng SF, Warren RM, Butler RC, Cromey MG (2014) he influence of growth stage of different cereal species on host susceptibility to Gaeumannomyces graminis var. tritici and on Pseudomonas populations in the rhizosphere. Australas Plant Pathol. doi:10.1007/s13313-014-0324-5
Vojinović ZD (1972) Antagonists from soil and rhizosphere to phytopathogens. Final technical report., Institute of soil science, Beograd
Vojinović ŽD (1973) The influence of micro-organisms following Ophiobolus graminis Sacc. on its further pathogenicity. EPPO Bulletin: 91–101
Weller DM (1983) Colonization of wheat roots by a fluorescent pseudomonad suppressive to take-all. Phytopathology 73:1548–1553
Weller DM (2007) Pseudomonas biocontrol agents of soilborne pathogens: looking back over 30 years. Phytopathology 97:250–256. doi:10.1094/phyto-97-2-0250
Weller DM, Cook RJ (1983) Suppression of take-all of wheat by seed treatments with fluorescent pseudomonads. Phytopathology 73:463–469
Weller DM, Zhang BX, Cook RJ (1985) Application of a rapid screening test for selection of bacteria suppressive to take-all of wheat. Plant Dis 69:710–713
Weller DM, Raaijmakers JM, Gardener BBM, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40:309–348
Whipps JM (1997a) Developments in the biological control of soil-borne plant pathogens. Adv Bot Res 26:1–134
Whipps JM (1997b) Ecological considerations involved in commercial development of bioligical control agents for soil-borne diseases. In: van Elsas JD, Trevors JT, Wellington EMH (eds) Modern soil microbiology. Marcel Dekker, Inc., New York, pp 525–548
White TJ, Bruns T, Lee S, Tayler J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, California, pp 315–322
Widmer F, Seidler RJ, Gillevet PM, Watrud LS, Giovanni GDD (1998) A highly selective pcr protocol for detecting 16S rRNA genes of the genus Pseudomonas (Sensu Stricto) in environmental samples. Appl Environ Microbiol 64:2545–2553
Wong PTW, Mead JA, Holley MP (1996) Enhanced field control of wheat take-all using cold tolerant isolates of Gaeumannomyces graminis var. graminis and Phialophora sp. (lobed hyphopodia). Plant Pathol 45:285–293
Yarham DJ (1981) Practical aspects of epidemiology and control. In: Asher MJC, Shipton PJ (eds) Biology and control of take-all. Academic, London, pp 353–384
Zriba N, Sherwood JE, Mathre DE (1999) Characterization and effectiveness of Phialophora spp. isolated from a Montana take-all suppressive soil in controlling take-all disease of wheat. Can J Plant Pathol Rev 21:110–118
Acknowledgments
The authors would like to thank South Australia Research & Development Institute, Adelaide for analysing soil Ggt DNA concentrations and the Ministry of Business, Innovation and Employment (MBIE) (Programme LINX0304) for funding the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Simon Jeffery.
Rights and permissions
About this article
Cite this article
Chng, S., Cromey, M.G., Dodd, S.L. et al. Take-all decline in New Zealand wheat soils and the microorganisms associated with the potential mechanisms of disease suppression. Plant Soil 397, 239–259 (2015). https://doi.org/10.1007/s11104-015-2620-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2620-4