Abstract
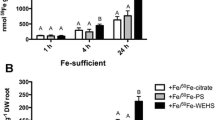
It is well known that in the rhizosphere soluble Fe sources available for plants are mainly a mixture of complexes between the micronutrient and organic ligands such as organic acids and phytosiderophores (PS) released by roots, microbial siderophores as well as fractions of humified organic matter. In the present work, mechanisms of Fe acquisition operating at the leaf level of plants fed with different Fe-complexes were investigated at the micro-analytical, physiological and molecular levels. Fe-deficient tomato plants (Solanum Lycopersicum L., cv. ‘Marmande’) were fed for 24 h with a solution (pH 7.5) containing 1 µM Fe as Fe-PS, Fe-citrate or Fe-WEHS. Thereafter, leaf tissue was used for the visualization of Fe distribution, measurements of Fe content, reduction and uptake, and evaluation of expression of Fe-chelate reductase (LeFRO1), Fe-transporter (LeIRT1) and Ferritin (Ferritin2) genes. Leaf discs isolated from Fe-deficient plants treated for 24 h with Fe-WEHS developed higher rates of translocation, Fe-chelate reduction and 59Fe uptake as compared to plants supplied with Fe-citrate or Fe-PS. Leaves of plants treated with Fe-WEHS also showed higher transcript levels of LeFRO1, LeIRT1 and Ferritin2 genes with respect to plants fed with the other Fe-sources. Data obtained support the idea that the efficient use of Fe complexed to WEHS-like humic fractions involves, at least in part, also the activation of Fe-acquisition mechanisms operating at the leaf level.





Similar content being viewed by others
Abbreviations
- PS:
-
Phytosiderophores
- WEHS:
-
Water-extractable humic fraction
- µ-XRF:
-
Micro x-ray fluorescence
References
Aiken GR, Thurman EM, Malcolm R (1979) Comparison of XAD macroporous resin for the concentration of fulvic acid from aqueous solution. Anal Chem 51:1799–1803
Astolfi S, Cesco S, Zuchi G et al (2006) Sulphur starvation reduces phytosiderophores release by Fe-deficient barley plants. Soil Sci Plant Nutr 52:43–48
Bienfait HF, Van den Briel W, Mesland-Mul NT (1985) Free space iron pools in roots: generation and mobilization. Plant Physiol 78:596–600
Briat JF, Curie C, Gaymard F (2007) Iron utilization and metabolism in plants. Curr Op Plant Biol 10:276–282
Chaney RL, Brown JC, Tiffin LO (1972) Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol 50:208–213
Connolly EL, Fett JP, Guerinot ML (2002) Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 14:1347–1357
Connolly EL, Campbell NH, Grotz N et al (2003) Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron uncovers posttranscriptional control. Plant Physiol 133:1102–1110
Cesco S, Römheld V, Varanini Z et al (2000) Solubilization of iron by water-extractable humic-substances. J Plant Nutr Soil Sci 163:285–290
Cesco S, Nikolic M, Römheld V et al (2002) Uptake of 59Fe from soluble 59Fe-humate complexes by cucumber and barley plants. Plant Soil 241:121–128
Cesco S, Rombolà AD, Tagliavini M et al (2006) Phytosiderophores released by graminaceous species promote 59Fe uptake in citrus. Plant Soil 287:223–233
Chen Y (1996) Organic matter reactions involving micronutrients in soils and their effect on plants. In: Piccolo A (ed) Humic substances in terrestrial ecosystems. Elsevier Science B.V, Amsterdam, pp 507–529
González-Vallejo EB, Morales F, Cistué L, Abadía A, Abadía J (2000) Iron deficiency decreases the Fe(III)-chelate reducing activity of leaf protoplasts. Plant Physiol 122:337–344
Hoerdt W, Römheld V, Winkelmann G (2000) Fusarinines and dimerum acid, mono- and dihydroxamate siderophores from Penicillum chrysogenum, improve iron utilization by strategy I and strategy II plants. Biometals 13:37
Howe JA, Choi YH, Loeppert RH et al (1999) Column chromatography and verification of phytosiderophores by phenylisothiocyanate derivatization and UV detection. J Chromatog. 841:155–164
Jimenéz S, Morales F, Abadia A, Abadia J, Moreno MA, Gocorcena Y (2009) Elemental 2-D mapping and changes in leaf iron and chlorophyll in response to iron re-supply in iron-deficient GF 677 peach-almond hybrid. Plant Soil 315:93–106
Jones DL (1998) Organic acids in the rhizosphere–A critical review. Plant Soil 205:25–44
Larbi A, Morales F, López-Millán A et al (2001) Technical advance: Reduction of Fe(III)-chelates by mesophyll leaf disks of sugar beet. Multi-component origin and effects of Fe deficiency. Plant Cell Physiol 42:94–105
Lindsay WL (1974) Role of chelation in the micronutrient availability. In: Carson EW (ed) The plant root and its environment. University Press of Virginia, Charlottesville, pp 507–524
Lindsay WL, Schwab AP (1982) The chemistry of iron in soils and its availability to plants. J Plant Nutr 5:821–840
López-Millán AF, Morales F, Abadia A et al (2001) Changes induced by Fe deficiency and Fe resupply in the organic acid metabolism of sugar beet (Beta vulgaris) leaves. Physiol Plant 112:31–38
López-Millán AF, Morales F, Gogorcena Y et al (2009) Metabolic responses in iron deficient tomato plants. J Plant Physiol 166:375–384
Maniatis T, Sambrook J, Fritsch EF (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Ma JF, Taketa S, Chang Y et al (1999) Genes controlling hydroxylations of phytosiderophores are located on different chromosomes in barley (Hordeum vulgare L.). Planta 207:590–597
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London
Mengel K, Kirkby E, Kosegarten H et al (2001) Iron. In Mineral Nutrition. Kluwer, Dordrecht, pp 553–571
Nikolic M, Römheld V (1999) Mechanism of Fe uptake by the leaf symplast: is Fe inactivation in leaf a cause of Fe deficiency chlorosis? Plant Soil 215:229–237
Nikolic M, Cesco S, Varanini Z et al (2007) Short-term interactions between nitrate and iron nutrition in cucumber. Funct Plant Biol 34:402–408
Pandeya SB, Singh AC, Dhar P (1998) Influence of fulvic acid on transport of iron in soils and uptake by paddy seedlings. Plant Soil 198:117
Pinton R, Cesco S, De Nobili M et al (1998) Water and pyrophosphate-extractable humic substances as a source of iron for Fe-deficient cucumber plants. Biol Fert Soil 26:23–27
Pinton R, Cesco S, Santi S et al (1999) Water-extractable humic substances enhance iron deficiency responses by Fe-deficient cucumber plants. Plant Soil 210:145–157
Ritz C, Spiess AN (2008) qpcR: an R package for sigmoidal model selection in quantitative real-time polymerase chain reaction analysis. Bioinform 24:1549–1551
Römheld V, Marschner H (1986a) Evidence for a specific uptake system for iron phytosiderophores in roots of grasses. Plant Physiol 80:175–180
Römheld V, Marschner H (1986b) Mobilization of iron in the rizosphere of different plant species. In: Tinker B, Laüchli A (eds) Advances in plant nutrition. Praeger Scientific, New York, pp 155–204
Sugiura Y, Tanaka H, Miro Y et al (1981) Structure, properties and transport mechanism of iron(III) complex of mugineic acid, a possible phytosiderophore. J Am Chem Soc 103:6979–6982
Tomasi N, De Nobili M, Varanini Z et al (2007) Use efficiency of natural iron complexes by tomato plants. International Symposium “Rhizosphere 2”, Montpellier, France, 145, P-1075
Terzano R, Al Chami Z, Vekemans B et al (2008) Zinc distribution and speciation within rocket plants (Eruca vesicaria L. Cavalieri) grown on a polluted soil amended with compost as determined by XRF microtomography and micro-XANES. J Agric Food Chem 56:3222–3231
Varanini Z, Pinton R (2006) Plant-Soil Relationship: Role of Humic Substances in Iron Nutrition. In: Barton LL, Abadía J (eds) Iron nutrition in plants and rhizospheric microorganisms. Springer Verlag, Heidelberg, pp 153–168
Vekemans B, Janssens K, Vincze L et al (1994) Analysis of X-ray spectra by iterative least squares (AXIL)–New developments. X-Ray Spectrom 23:278–285
von Wirén N, Mori S, Marschner H et al (1994) Iron inefficiency in maize mutant ys1 (Zea mays L. cv Yellow-Stripe) is caused by a defect in uptake of iron phytosiderophores. Plant Physiol 106:71–77
Walter A, Pich A, Scholz G et al (1995) Diurnal variation in release of phytosiderophores and in concentration of phytosiderophores and nicotianamine in roots and shoots of barley. J Plant Physiol 147:191–196
Wu L, Zhang J, Miller DD et al (2008) Iron enrichment in rice (Oriza sativa L.) grain using a foliar high-efficiency iron containing fertilizer in a crop-soil system. Abstract of 14th International Symposium on Iron Nutrition and Interaction in Plants, Beijing, China, 141
Zaharieva T, Römheld V (2000) Specific Fe2+ uptake system in strategy I plants inducible under Fe deficiency. J Plant Nutr 23:1733–1744
Zancan S, Cesco S, Ghisi R (2006) Effect of UV-B radiation on iron content and distribution in maize plants. Environm Exp Bot 55:266–272
Zhang FS, Römheld V, Marschner H (1991) Role of the root apoplasm for iron acquisition by wheat plants. Plant Physiol 97:1302–1305
Acknowledgements
Research was supported by grant from Italian M.U.R.S.T.. We thank: Dr. Adamo Domenico Rombolà (University of Bologna) for performing PS analysis, Dr. J.F. Ma (Kagawa University) for providing purified epi-HMA, Prof Volker Römheld (Hohenheim University) for providing barley seeds cv ‘Europa’, Dr. Filip Pošćić (University of Udine) for performing ICP-AES analyses. Synchrotron experiments at HASYLAB were financially supported by the European Community-Research Infrastructure Action under the FP6 “Structuring the European Research Area” Program (Integrating Activity on Synchrotron and Free Electron Laser Science; Contract RII3-CT-2004-506008). This part of the research was also performed as part of the “Interuniversity Attraction Poles” programme financed by the Belgian government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jian Feng Ma.
Rights and permissions
About this article
Cite this article
Tomasi, N., Rizzardo, C., Monte, R. et al. Micro-analytical, physiological and molecular aspects of Fe acquisition in leaves of Fe-deficient tomato plants re-supplied with natural Fe-complexes in nutrient solution. Plant Soil 325, 25–38 (2009). https://doi.org/10.1007/s11104-009-0069-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-009-0069-z