Abstract
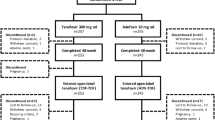
Background Tenofovir disoproxil fumarate (TDF) has demonstrated potent antiviral activity against hepatitis B virus (HBV) in clinical trials. Although its efficacy has been demonstrated in Caucasian populations, TDF has not previously been studied in Korean patients who present the predominance of HBV genotype C and of vertical or perinatal transmission. Objective The aim of this study was to evaluate the efficacy of TDF in Korean chronic hepatitis B (CHB) patients in real-life practice, and to determine the clinical variables that contribute to virologic response.Setting Large academic medical center in Korea. Method We retrospectively investigated the efficacy of TDF treatment for more than 6 months in 151 nucleos(t)ide-naïve CHB patients. Main outcome measure The primary endpoint was a virologic response (VR), defined as an HBV DNA level of <12 IU/mL. Secondary endpoints were rates of alanine aminotransaminase (ALT) normalization, hepatitis B e antigen (HBeAg) seroconversion, virologic breakthrough, and safety. Results All patients were the genotype C2. The median duration of TDF treatment was 13 months (range 7–18 months). Ninety-two (61.0 %) patients were HBeAg positive. The mean pre-treatment HBV DNA level was 6.34 ± 1.42 log10 IU/mL. Among the 131 patients with elevated ALT levels at baseline, 128 (97.7 %) patients achieved ALT normalization during TDF treatment. VR was achieved in 97 (64.2 %) patients. The cumulative rates of VR at 6, 9, 12, and 18 months were 47.0, 59.4, 67.9, and 69.3 %, respectively. Among the 92 HBeAg-positive patients, 14 (15.2 %) patients achieved HBeAg seroconversion. In multivariate analysis, absolute HBV DNA levels at baseline (P < 0.001; OR 0.529; 95 % CI 0.560–0.744) and HBeAg positivity (P = 0.015; OR 0.731; 95 % CI 0.615–0.869) were significantly associated with VR. Virologic breakthrough was observed in four patients. These four patients had poor adherence to TDF. Most of the adverse events were mild in severity. No significant changes were observed in serum creatinine and phosphorus levels. Conclusions TDF was effective and well tolerated in Korean genotype C CHB patients in real life practice, consistent with larger registration trials. The absolute HBV DNA levels at baseline and HBeAg positivity were significantly associated with VR.



Similar content being viewed by others
References
Evans AA, London WT, Gish RG, Cohen C, Block TM. Chronic HBV infection outside treatment guidelines: Is treatment needed? Antivir Ther. 2013;18(2):229–35.
Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73.
Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, et al. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology. 2011;141(4):1240–8, 8 e1-2.
Wong VW, Chan SL, Mo F, Chan TC, Loong HH, Wong GL, et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol. 2010;28(10):1660–5.
Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58(1):98–107.
Lai CL, Yuen MF. Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy. Hepatology. 2013;57(1):399–408.
Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52(3):886–93.
Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–75.
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661–2.
EASL clinical practice guidelines. Management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167–85.
Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359(23):2442–55.
Gordon SC, Krastev Z, Horban A, Petersen J, Sperl J, Dinh P, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58(2):505–13.
Kitrinos KM, Corsa A, Liu Y, Flaherty J, Snow-Lampart A, Marcellin P, et al. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2014;59(2):434–42.
Pan CQ, Hu KQ, Tsai N. Long-term therapy with nucleoside/nucleotide analogues for chronic hepatitis B in Asian patients. Antivir Ther. 2013;18(7):841–52.
Lampertico P, Soffrendi R, Vigano M, Yurdaydin C, Idilman R, Papatheodoridis GV, et al. Tenofovir monotherapy suppressed viral suppression in most field practice, treatment-naïve patients with chronic hepatitis B followed for 3 years in a multicenter European study. Hepatology. 2012;56(S1):389A.
Marcellin P, Zoulim F, Causse X, Hézode C, Larrey D, Pageaux G, et al. High efficacy and safety of tenofovir DF in 441 naïve and NUC-experienced chronic hepatitis B patients: a real life multicenter prospective cohort study. J Hepatol. 2012;56(S2):S210.
Petersen J, Heyne R, Mauss S, Schlaak J, Schiffelholz W, Eisenbach C, et al. Tenofovir DF for chronic hepatitis B patients in filed practice—results from the GEMINIS German multicenter observational study. J Hepatol. 2012;56(S2):S212–3.
Pan CQ, Trinh H, Yao A, Bae H, Lou L, Chan S, et al. Efficacy and safety of tenofovir disoproxil fumarate in Asian-Americans with chronic hepatitis B in community settings. PLoS ONE. 2014;9(3):e89789.
Zoutendijk R, Reijnders JG, Brown A, Zoulim F, Mutimer D, Deterding K, et al. Entecavir treatment for chronic hepatitis B: adaptation is not needed for the majority of naive patients with a partial virological response. Hepatology. 2011;54(2):443–51.
Yuen MF, Seto WK, Fung J, Wong DK, Yuen JC, Lai CL. Three years of continuous entecavir therapy in treatment-naive chronic hepatitis B patients: VIRAL suppression, viral resistance, and clinical safety. Am J Gastroenterol. 2011;106(7):1264–71.
Ono A, Suzuki F, Kawamura Y, Sezaki H, Hosaka T, Akuta N, et al. Long-term continuous entecavir therapy in nucleos(t)ide-naive chronic hepatitis B patients. J Hepatol. 2012;57(3):508–14.
Shin JW, Jung SW, Park BR, Kim CJ, Eum JB, Kim BG, et al. Prediction of response to entecavir therapy in patients with HBeAg-positive chronic hepatitis B based on on-treatment HBsAg, HBeAg and HBV DNA levels. J Viral Hepat. 2012;19(10):724–31.
Gish RG, Lok AS, Chang TT, de Man RA, Gadano A, Sollano J, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133(5):1437–44.
Shouval D, Lai CL, Chang TT, Cheinquer H, Martin P, Carosi G, et al. Relapse of hepatitis B in HBeAg-negative chronic hepatitis B patients who discontinued successful entecavir treatment: the case for continuous antiviral therapy. J Hepatol. 2009;50(2):289–95.
Sherman M, Yurdaydin C, Simsek H, Silva M, Liaw YF, Rustgi VK, et al. Entecavir therapy for lamivudine-refractory chronic hepatitis B: improved virologic, biochemical, and serology outcomes through 96 weeks. Hepatology. 2008;48(1):99–108.
Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, et al. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51(2):422–30.
Gish RG, Chang TT, Lai CL, de Man R, Gadano A, Poordad F, et al. Loss of HBsAg antigen during treatment with entecavir or lamivudine in nucleoside-naive HBeAg-positive patients with chronic hepatitis B. J Viral Hepat. 2010;17(1):16–22.
Chu CJ, Hussain M, Lok AS. Quantitative serum HBV DNA levels during different stages of chronic hepatitis B infection. Hepatology. 2002;36(6):1408–15.
Lin B, Ha NB, Liu A, Trinh HN, Nguyen HA, Nguyen KK, et al. Low incidence of hepatitis B e antigen seroconversion in patients treated with oral nucleos(t)ides in routine practice. J Gastroenterol Hepatol. 2013;28(5):855–60.
Baran B, Soyer OM, Ormeci AC, Gokturk S, Evirgen S, Bozbey HU, et al. Efficacy of tenofovir in patients with Lamivudine failure is not different from that in nucleoside/nucleotide analogue-naive patients with chronic hepatitis B. Antimicrob Agents Chemother. 2013;57(4):1790–6.
Pol S, Lampertico P. First-line treatment of chronic hepatitis B with entecavir or tenofovir in ‘real-life’ settings: from clinical trials to clinical practice. J Viral Hepat. 2012;19(6):377–86.
Hongthanakorn C, Chotiyaputta W, Oberhelman K, Fontana RJ, Marrero JA, Licari T, et al. Virological breakthrough and resistance in patients with chronic hepatitis B receiving nucleos(t)ide analogues in clinical practice. Hepatology. 2011;53(6):1854–63.
Lok AS, Trinh H, Carosi G, Akarca US, Gadano A, Habersetzer F, et al. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naive patients with chronic hepatitis B. Gastroenterology. 2012;143(3):619–28 e1.
Chotiyaputta W, Hongthanakorn C, Oberhelman K, Fontana RJ, Licari T, Lok AS. Adherence to nucleos(t)ide analogues for chronic hepatitis B in clinical practice and correlation with virological breakthroughs. J Viral Hepat. 2012;19(3):205–12.
Acknowledgments
This study was supported by Priority Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2009-0094050).
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None of the authors has any conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Kim, J.H., Jung, S.W., Byun, S.S. et al. Efficacy and safety of tenofovir in nucleos(t)ide-naïve patients with genotype C chronic hepatitis B in real-life practice. Int J Clin Pharm 37, 1228–1234 (2015). https://doi.org/10.1007/s11096-015-0193-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-015-0193-1