Abstract
Purpose
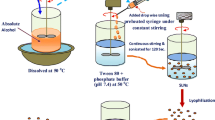
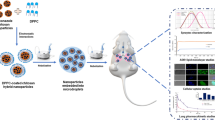
In this study, cationic mannosylated nanostructured lipid carriers (Man-NLCs) were developed for the targeted delivery of rifampicin to alveolar macrophages.
Methods
Rifampicin loaded Man-NLCs (RFP-Man-NLCs) and rifampicin loaded unmodified nanostructured lipid carriers (REP-NLCs) were prepared using thin film homogenization method and characterized by particle size, polydispersity index, zeta potential, transmission electron microscopy, encapsulation efficiency, pharmacokinetics, biodistribution, cell specific targeting, cytotoxicity and inflammatory response.
Results
RFP-Man-NLCs and REP-NLCs obtained displayed a size distribution around 160 nm (PDI <0.30) with positive charges of approximately 30 mV. The encapsulation efficiency of RFP was above 90%. In the biodistribution study, both RFP-Man-NLCs and RFP-NLCs, compared with the commercially available rifampicin solution, displayed superior lung-targeting ability. Compared to REP-NLCs, RFP-Man-NLCs exhibited significantly higher uptake efficiency in NR8383 cells and alveolar macrophages, which achieved cell-specific targeting. In addition, RFP-Man-NLCs were demonstrated to be a safe formulation with minimum toxicity and no inflammatory response.
Conclusions
RFP-Man-NLCs provided an alternative strategy for selectively delivering rifampicin to alveolar macrophages.






Similar content being viewed by others
Abbreviations
- AMs:
-
Alveolar macrophages
- AUC:
-
Area under the curve
- CLz:
-
Plasma clearance
- Cmax :
-
maximum concentration
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DDAB:
-
Dimethyldioctadecylammonium bromide
- DMSO:
-
Dimethyl sulfoxide
- EPC:
-
Purified ovolecithin
- FITC-DHPE:
-
Fluorescein isothiocyanate labeled DHPE
- MCT:
-
Medium chain triglyceride
- MRT:
-
Mean retention time
- MTT:
-
3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide
- OCT:
-
Octadecylamine
- PDI:
-
Polydispersity index
- REP-NLCs:
-
Rifampicin loaded nanostructured lipid carriers
- RFP:
-
Rifampicin
- RFP Solution:
-
Rifampicin commercial solution
- RFP-Man-NLCs:
-
Cationic mannosylated rifampicin nanostructured lipid carriers
- t1/2z :
-
Half-life
- TEM:
-
Transmission electron microscopy
References
Aal AMA, El-Mashad N, Magdi D. Tuberculosis problem in Dakahlia Governorate, Egypt. SAARC J Tuberc Lung Dis HIV/AIDS. 2013;10:43–9.
Fauci AS. Infectious diseases: considerations for the 21st century. Clin Infect Dis. 2001;32:675–85.
Bermudez LE. Use of liposome preparation to treat mycobacterial infections. Immunobiology. 1994;191:578–83.
Vyas S, Kannan M, Jain S, Mishra V, Singh P. Design of liposomal aerosols for improved delivery of rifampicin to alveolar macrophages. Int J Pharm. 2004;269:37–49.
Natarajan K, Kundu M, Sharma P, Basu J. Innate immune responses to< i> M. tuberculosis</i> infection. Tuberculosis. 2011;91:427–31.
McKinney JD, zu Bentrup KH, Muñoz-Elías EJ, Miczak A, Chen B, Chan W-T, et al. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature. 2000;406:735–8.
Zahrt TC. Molecular mechanisms regulating persistent< i> Mycobacterium tuberculosis</i> infection. Microbes Infect. 2003;5:159–67.
Wijagkanalan W, Kawakami S, Takenaga M, Igarashi R, Yamashita F, Hashida M. Efficient targeting to alveolar macrophages by intratracheal administration of mannosylated liposomes in rats. J Control Release. 2008;125:121–30.
Chuan J, Li Y, Yang L, Sun X, Zhang Q, Gong T, et al. Enhanced rifampicin delivery to alveolar macrophages by solid lipid nanoparticles. J Nanoparticle Res. 2013;15:1–9.
Yee D, Valiquette C, Pelletier M, Parisien I, Rocher I, Menzies D. Incidence of serious side effects from first-line antituberculosis drugs among patients treated for active tuberculosis. Am J Respir Crit Care Med. 2003;167:1472–7.
Mohr JF, McKinnon PS, Peymann PJ, Kenton I, Septimus E, Okhuysen PC. A retrospective, comparative evaluation of dysglycemias in hospitalized patients receiving gatifloxacin, levofloxacin, ciprofloxacin, or ceftriaxone. Pharmacother J Hum Pharmacol Drug Ther. 2005;25:1303–9.
Shi S, Han L, Gong T, Zhang Z, Sun X. Systemic delivery of microRNA‐34a for cancer stem cell therapy. Angew Chem. 2013;125:3993–7.
Braeckmans K, Buyens K, Bouquet W, Vervaet C, Joye P, Vos FD, et al. Sizing nanomatter in biological fluids by fluorescence single particle tracking. Nano Lett. 2010;10:4435–42.
Li S, Tseng W, Stolz DB, Wu S, Watkins S, Huang L. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther. 1999;6:585–94.
Ishiwata H, Suzuki N, Ando S, Kikuchi H, Kitagawa T. Characteristics and biodistribution of cationic liposomes and their DNA complexes. J Control Release. 2000;69:139–48.
Han J, Wang Q, Zhang Z, Gong T, Sun X. Cationic bovine serum albumin based self assembled nanoparticles as siRNA delivery vector for treating lung metastatic cancer. Small. 2014;10:524–35.
Kutscher HL, Chao P, Deshmukh M, Singh Y, Hu P, Joseph LB, et al. Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats. J Control Release. 2010;143:31–7.
Deshmukh M, Kutscher HL, Gao D, Sunil VR, Malaviya R, Vayas K, et al. Biodistribution and renal clearance of biocompatible lung targeted poly (ethylene glycol)(PEG) nanogel aggregates. J Control Release. 2012;164:65–73.
Lawlor C, Kelly C, O’Leary S, O’Sullivan M, Gallagher P, Keane J, et al. Cellular targeting and trafficking of drug delivery systems for the prevention and treatment of MTb. Tuberculosis. 2011;91:93–7.
Briones E, Isabel Colino C, Lanao JM. Delivery systems to increase the selectivity of antibiotics in phagocytic cells. J Control Release. 2008;125:210–27.
Chellat F, Merhi Y, Moreau A, Yahia LH. Therapeutic potential of nanoparticulate systems for macrophage targeting. Biomaterials. 2005;26:7260–75.
Largent B, Walton K, Hoppe C, Lee Y, Schnaar R. Carbohydrate-specific adhesion of alveolar macrophages to mannose-derivatized surfaces. J Biol Chem. 1984;259:1764–9.
Li P, Chen S, Jiang Y, Jiang J, Zhang Z, Sun X. Dendritic cell targeted liposomes–protamine–DNA complexes mediated by synthetic mannosylated cholestrol as a potential carrier for DNA vaccine. Nanotechnology. 2013;24:295101.
Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–84.
Guo Y, Liu X, Sun X, Zhang Q, Gong T, Zhang Z. Mannosylated lipid nano-emulsions loaded with lycorine-oleic acid ionic complex for tumor cell-specific delivery. Theranostics. 2012;2:1104.
Melo L, Queiroz R, Queiroz M. Automated determination of rifampicin in plasma samples by in-tube solid-phase microextraction coupled with liquid chromatography. J Chromatogr B. 2011;879:2454–8.
Zhao D, Gao ZD, Han DE, Li N, Zhang YJ, Lu Y, et al. Influence of rifampicin on the pharmacokinetics of salvianolic acid B may involve inhibition of organic anion transporting polypeptide (oatp) mediated influx. Phytother Res. 2012;26:118–21.
Cui Z, Han S-J, Huang L. Coating of mannan on LPD particles containing HPV E7 peptide significantly enhances immunity against HPV-positive tumor. Pharm Res. 2004;21:1018–25.
Hattori Y, Kawakami S, Lu Y, Nakamura K, Yamashita F, Hashida M. Enhanced DNA vaccine potency by mannosylated lipoplex after intraperitoneal administration. J Gene Med. 2006;8:824–34.
Chakravarthy KV, Davidson BA, Helinski JD, Ding H, Law W-C, Yong K-T, et al. Doxorubicin-conjugated quantum dots to target alveolar macrophages and inflammation. Nanomed Nanotechnol Biol Med. 2011;7:88–96.
Jones B, Dickinson P, Gumbleton M, Kellaway I. Lung surfactant phospholipids inhibit the uptake of respirable microspheres by the alveolar macrophage NR8383. J Pharm Pharmacol. 2002;54:1065–72.
Diab R, Brillault J, Bardy A, Gontijo AVL, Olivier JC. Formulation and in vitro characterization of inhalable polyvinyl alcohol-free rifampicin-loaded PLGA microspheres prepared with sucrose palmitate as stabilizer: efficiency for ex vivo alveolar macrophage targeting. Int J Pharm. 2012;436:833–9.
Hirota K, Hasegawa T, Hinata H, Ito F, Inagawa H, Kochi C, et al. Optimum conditions for efficient phagocytosis of rifampicin-loaded PLGA microspheres by alveolar macrophages. J Control Release. 2007;119:69–76.
Uner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): their benefits as colloidal drug carrier systems. Die Pharm Int J Pharm Sci. 2006;61:375–86.
Müller R, Radtke M, Wissing S. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242:121–8.
Li M, Zheng Y, Shan F-Y, Zhou J, Gong T, Zhang Z-R. Development of ionic-complex-based nanostructured lipid carriers to improve the pharmacokinetic profiles of breviscapine. Acta Pharmacol Sin. 2013;34:1108–15.
Illumand L, Davis S. The targeting of drugs parenterally by use of microspheres. PDA J Pharm Sci Technol. 1982;36:242–8.
Rappand R, Bivins B. Final in-line filtration: removal of contaminants from IV fluids and drugs. Hosp Formul. 1983;18:1124–8.
Wilkinsand D, Myers P. Studies on the relationship between the electrophoretic properties of colloids and their blood clearance and organ distribution in the rat. Br J Exp Pathol. 1966;47:568.
Fidler I, Raz A, Fogler W, Kirsh R, Bugelski P, Poste G. Design of liposomes to improve delivery of macrophage-augmenting agents to alveolar macrophages. Cancer Res. 1980;40:4460–6.
Vroman L, Adams A, Fischer G, Munoz P. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood. 1980;55:156–9.
Göppertand T, Müller R. Adsorption kinetics of plasma proteins on solid lipid nanoparticles for drug targeting. Int J Pharm. 2005;302:172–86.
Buyens K, Meyer M, Wagner E, Demeester J, De Smedt SC, Sanders NN. Monitoring the disassembly of siRNA polyplexes in serum is crucial for predicting their biological efficacy. J Control Release. 2010;141:38–41.
Buyens K, De Smedt SC, Braeckmans K, Demeester J, Peeters L, van Grunsven LA, et al. Liposome based systems for systemic siRNA delivery: stability in blood sets the requirements for optimal carrier design. J Control Release. 2012;158:362–70.
Tasduq S, Singh K, Satti N, Gupta D, Suri K, Johri R. Terminalia chebula (fruit) prevents liver toxicity caused by sub-chronic administration of rifampicin, isoniazid and pyrazinamide in combination. Hum Exp Toxicol. 2006;25:111–8.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was funded by the National S&T Major Project of China (Grant No. 2012ZX09304004001) and the National Basic Research Program of China (No. 2013CB932504). The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Song, X., Lin, Q., Guo, L. et al. Rifampicin Loaded Mannosylated Cationic Nanostructured Lipid Carriers for Alveolar Macrophage-specific Delivery. Pharm Res 32, 1741–1751 (2015). https://doi.org/10.1007/s11095-014-1572-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1572-3