ABSTRACT
Purpose
To assess the feasibility of transdermal macromolecule delivery using novel laser-engineered dissolving microneedles (MNs) prepared from aqueous blends of 20% w/w poly(methylvinylether maleic anhydride) (PMVE/MA) in vitro and in vivo.
Methods
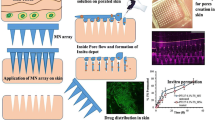
Micromoulding was employed to prepare insulin-loaded MNs from aqueous blends of 20% w/w PMVE/MA using laser-engineered moulds. To investigate conformational changes in insulin loaded into MNs, circular dichroism spectra were obtained. In vitro drug release studies from MNs across neonatal porcine skin were performed using Franz diffusion cells. The in vivo effect of MNs was assessed by their percutaneous administration to diabetic rats and measurement of blood glucose levels.
Results
MNs loaded with insulin constituted exact counterparts of mould dimensions. Circular dichroism analysis showed that encapsulation of insulin within polymeric matrix did not lead to change in protein secondary structure. In vitro studies revealed significant enhancement in insulin transport across the neonatal porcine skin. Percutaneous administration of insulin-loaded MN arrays to rats resulted in a dose-dependent hypoglycaemic effect.
Conclusion
We demonstrated the efficacy of MNs prepared from aqueous blends of PMVE/MA in transdermal delivery of insulin. We are currently investigating the fate of the delivered insulin in skin and MN-mediated delivery of other macromolecules.







Similar content being viewed by others
Abbreviations
- BGL:
-
blood glucose level
- BSA:
-
bovine serum albumin
- CD:
-
circular dichroism
- MN:
-
microneedle
- PMVE/MA:
-
poly(methylvinylether) maleic anhydride
- SC :
-
stratum corneum
REFERENCES
Brown L. Commercial challenges of protein drug delivery. Expert Opin Drug Del. 2005;2(1):29–42.
Kumar T, Soppimath K, Nachaegari S. Novel delivery technologies for protein and peptide therapeutics. Curr Pharm Biotechnol. 2006;7:261–76.
Banga AK. Therapeutic Peptides and Proteins: Formulation, Processing and Delivery Systems. Boca Raton: CRC Press; 2006.
Gupta H, Sharma A. Recent trends in protein and peptide drug delivery systems. Asian J Pharm. 2009;3(2):69–75.
Benson H, Namjoshi S. Proteins and peptides: Strategies for delivery to and across the skin. J Pharm Sci. 2008;97(9):3591–610.
Chaturvedula A, Joshi D, Anderson C, Morris R, Sembrowich W, Banga A. In vivo iontophoretic delivery and pharmacokinetics of salmon calcitonin. Int J Pharm. 2005;97(1–2):190–6.
Medi B, Singh J. Electronically facilitated transdermal delivery of human parathyroid hormone (1–34). Int J Pharm. 2003;263:25–33.
Park E, Werner J, Smith N. Ultrasound mediated transdermal insulin delivery in pigs using a lightweight transducer. Pharm Res. 2007;24(7):1396–401.
Badkar A, Smith A, Eppstein J, Banga A. Transdermal delivery of interferon alpha-2B using microporation and iontophoresis in hairless rats. Pharm Res. 2007;24:1389–95.
Fang JY, Lee WR, Shen SC, Wang HY, Fang CL, Hu CH. Transdermal delivery of macromolecules by erbium:YAG laser. J Control Release. 2004;100:75–85.
Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56(5):581–7.
Kaushik S, Allen H, Donald D, McAllister D, Smitra S, Allen M, et al. Lack of pain associated with microfabricated microneedles. Anesth Analg. 2001;92:502–4.
Martanto W, Davis SP, Holiday NR, Wang J, Gill H, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm Res. 2004;21(6):947–52.
Donnelly RF, Morrow DIJ, McCarron PA, Woolfson AD, Morrissey A, Juzenas P, et al. Microneedle-mediated intradermal delivery of 5-aminolevulinic acid: Potential for enhanced topical photodynamic therapy. J Control Release. 2008;129:154–62.
Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19(1):63–70.
Davis S, Martanto W, Allen M, Prausnitz M. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52(5):909–15.
Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–24.
Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res. 2006;23(5):1008–19.
Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104:51–66.
Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–8.
Donnelly RF, Majithiya R, Singh R, Morrow D, Garland M, Demir YK, et al. Design, optimization and characterization of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm Res. 2011;28(1):41–57.
Henricus M, Johnon K, Banerje I. Investigation of insulin loaded self-assembled microtubulus for drug release. Bioconjug Chem. 2008;19:2394–400.
Bouchard M, Zurdo J, Nettleton E, Dobson C, Robinson C. Formation of insulin amyloid fibrils followed by FTIR and simultaneously with CD and electron microscopy. Protein Sci. 2000;9:1960–7.
Owens DR, Zinman B, Bolli G. Alternative routes of insulin delivery. Diabet Med. 2003;20:886–98.
Karande P, Jain A, Mitragotri S. Discovery of transdermal penetration enhancers by high-throughput screening. Nat Biotechnol. 2004;22(2):192–7.
Ito Y, Saeki A, Shiroyama K, Sugioka N, Takada K. Percutaneous absorption of interferon-alpha by self-dissolving micropiles. J Drug Target. 2008;16(3):243–9.
Langkjaer L, Brange J, Grodsky GM, Guy R. Iontophoresis of monomeric insulin analogues in vitro: effects of insulin charge and skin pretreatment. J Control Release. 1998;51:47–56.
Tezel A, Sens A, Mitragotri S. Description of transdermal transport of hydrophilic solutes during low-frequency sonophoresis based on a modified porous pathway model. J Pharm Sci. 2003;92(2):381–93.
Ito Y, Ohashi Y, Saeki A, Sugioka N, Takada K. Antihyperglycemic effect of insulin from self-dissolving micropiles in dogs Chem. Pharm Bull. 2008;56(3):243–6.
Cevc G. Transdermal drug delivery of insulin with ultradeformable carriers. Clin Pharmacokinet. 2003;42(5):461–74.
King M, Badea I, Solomon J, Kumar P, Gaspar K, Foldvari M. Transdermal delivery of insulin from novel biphasic lipid system in diabetic rats. Diab Technol The. 2002;4(4):479–88.
Levin G, Gershonowitz A, Sacks H, Stern M, Sherman A, Rudaev S, et al. Transdermal delivery of human growth hormone through RF-microchannels. Pharm Res. 2005;22(4):550–5.
Banga A. Theme section: Transdermal delivery of proteins. Pharm Res. 2007;24(7):1357–9.
Ito Y, Yamazaki T, Sugioka N, Takada K. Self-dissolving micropile array tips for percutaneous administration of insulin. J Mater Sci Mater M. 2010;21:835–41.
Donnelly RF, Morrow DIJ, Thakur RRS, Migalska K, McCarron PA, O'Mahony C, et al. Processing difficulties and instability of carbohydrate microneedle arrays. Drug Dev Ind Pharm. 2009;35:1242–54.
Ito Y, Eiji H, Atsushi S, Nobuyuki S, Kanji T. Feasibility of microneedles for percutaneous absorption of insulin. Euro J Pharm Sci. 2006;29(1):82–8.
Sullivan SP, Murthy N, Prausnitz MR. Minimally invasive protein delivery with rapidly dissolving polymer microneedles. Adv Mater. 2008;20:933–8.
Wangoo N, Suri C, Shekawat G. Interaction of gold nanoparticles with protein: A spectroscopic study to monitor protein conformational changes. App. Phys. Lett. 2008;92(13) ID 133104
Brandes N, Welzel P, Werner C, Kroh L. Adsorption-induced conformational changes of proteins onto ceramic particles: Differential scanning calorimetry and FTIR analysis. J Colloid Interf Sci. 2006;299(1):56–69.
Lougheed WD, Woulfe-Flanagan H, Clement JR, Albisser A. Insulin aggregation in artificial delivery systems. Diabetologia. 1980;19:1–9.
Pillai O, Borkute S, Sivaprasad N, Panchagnula R. Transdermal iontophoresis of insulin II. Physicochemical considerations. Int J Pharm. 2003;254:271–80.
Huang Y, Wu S. Stability of peptides during iontophoretic transdermal delivery. Int J Pharm. 1996;131:19–23.
Srinivasan V, Higuchi W, Sims S, Ghanem A, Behl C. Transdermal iontophoretic drug delivery: Mechanistic analysis and application to polypeptide delivery. J Pharm Sci. 1989;78(5):370–5.
International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use, ICH harmonised tripartite guideline—Validation of Analytical Procedures: Text and Methodology—Q2(R1), 2005
Kadima W, Ogendal L, Bauer R, Kaarsholm N, Brodersen K, Hansen JF, et al. The influence of ionic strength and pH on the aggregation properties of zinc-free insulin studied by static and dynamic laser light scattering. Biopolymers. 1993;33(11):1643–57.
Tokumoto S, Higo N, Sugibayashi K. Effect of electroporation and pH on iontophoretic transdermal delivery of human insulin. Int J Pharm. 2006;326:13–9.
Sadhale Y, Shah J. Biological activity of insulin in GMO gels and the effect of agitation Int. J Pharm. 1999;191:65–74.
ACKNOWLEDGMENTS
This work was supported by BBSRC grant number BB/E020534/1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Migalska, K., Morrow, D.I.J., Garland, M.J. et al. Laser-Engineered Dissolving Microneedle Arrays for Transdermal Macromolecular Drug Delivery. Pharm Res 28, 1919–1930 (2011). https://doi.org/10.1007/s11095-011-0419-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0419-4