Abstract
Purpose
The pharmacokinetics, analgesic efficacy, and irritancy potential of Buprederm™, a new transdermal delivery system of buprenorphine, was evaluated.
Methods
Single and multiple dose pharmacokinetic studies were conducted in mice and rabbits. The analgesic efficacy and skin irritation potential were determined by tail flick and writhing tests in mice and by the Draize dermal scoring system in rabbits.
Results
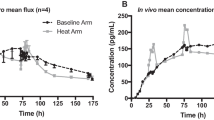
Fast absorption of buprenorphine into the bloodstream was observed in mice and rabbits after Buprederm™ application. The peak buprenorphine level in plasma was achieved between 1 and 24 h, and the effective therapeutic drug concentration was maintained for 72 h. No significant accumulation of buprenorphine was seen after multiple consecutive applications of patches to rabbits with a 4-day dosing interval. Buprederm™ induced prolongation of tail-flick latency in a dose- and time-dependent manner. Maximum analgesic effect was attained between 3 and 6 h and was maintained for 24 h after patch application. No skin irritation was demonstrated in rabbits after repeated Buprederm™ application.
Conclusions
Buprederm™ was shown to be efficacious by achieving the effective buprenorphine concentration in the blood and brain sufficient to maintain an analgesic effect for 72 h, and was also shown to be safe following multiple applications.




Similar content being viewed by others
Abbreviations
- BBB:
-
Blood–brain barrier
- BTDS:
-
Buprenorphine transdermal system
- HPLC:
-
High performance liquid chromatography
- IS:
-
Internal standard
- LC/MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- LOQ:
-
Limit of quantification
- ME:
-
Maximum effect
- MeOH:
-
Methanol
- MRM:
-
Multiple reaction monitoring
- P-gp :
-
P-glycoprotein
- PK/PD:
-
Pharmacokinetic/pharmacodynamic
- S.D.:
-
Standard deviation
- S.E.M.:
-
Standard error of means
- TDS:
-
Transdermal delivery system
References
H. Blumberg, U. Hoffmann, M. Mohadjer, and R. Scheremet. Sympathetic nervous system and pain: a clinical reappraisal. Behav. Brain Sci. 20(3):426–434 (1997).
D. A. Drossman, F. H. Creed, G. A. Fava, K. W. Olden, D. L. Patrick, and B. B. Toner. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterol. Int. 8:47–90 (1995).
J. Jailwala, T.F. Imperiale, and K. Kroenke. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann. Intern. Med. 133:135–147 (2000).
K. S. Lewis, K. J. Wipple, K. A. Michael, and E. J. Quebbeman. Effect of analgesic treatment on the physiological consequences of acute pain. Am. J. Hosp. Pharm. 51(12):1539–1554 (1994).
G. P. Joshi, and P. F. White. Management of acute and postoperative pain. Curr. Opin. Anaesthesiol. 14(4):417–421 (2001).
S. Suresh. Chronic and cancer pain management. Curr. Opin. Anaesthesiol. 17(3):253–259 (2004).
I. Gralow. Cancer pain: an update of pharmacological approaches in pain therapy. Curr. Opin. Anaesthesiol. 15(5):555–561 (2002).
S. Perrot. Management strategies for the treatment of non malignant chronic pain in the elderly. Psychol. Neuropsychiatr. Vieil. 4(3):163–170 (2006).
L. Brasseur. Review of current pharmacologic treatment of pain. Drugs 53(Suppl 2):10–17 (1997)(Review).
M. Krsiak. How to advance in treating pain with opioids: less myths—less pain. Cesk Fysiol. 53(1):34–38 (2004)(Review).
T. L. Schaer. Practice guidelines for transdermal opioids in malignant pain. Drugs 64(23):2629–2638 (2004)(Review).
P. R. Picard, M. R. Tramer, H. J. McQuay, and R. A. Moore. Analgesic efficacy of peripheral opioids (all except intra-articular): a qualitative systematic review of randomised controlled trials. Pain 72(3):309–18 (1997)(Review).
S. Grond, and T. Meuser. Weak opioids—an educational substitute for morphine? Curr. Opin. Anaesthesiol. 11(5):559–565 (1998).
E. C. Strain, M. L. Stitzer, I. A. Liebson, and G. E. Bigelow. Buprenorphine versus methadone in the treatment of opioid dependence: self-reports, urinalysis, and addiction severity index. J. Clin. Psychopharmacol. 16(1):58–67 (1996).
E. C. Strain, M. L. Stitzer, I. A. Liebson, and G. E. Bigelow. Comparison of buprenorphine and methadone in the treatment of opioid dependence. Am. J. Pharm. Sci. 83:126–130 (1994).
D. R. Jasinski, J. S. Pevnick, and J. D. Griffith. Human pharmacology and abuse potential of the analgesic buprenorphine. Arch. Gen. Psychiatry. 35:501–516 (1978).
E. Gorman. Fentanyl abuse and dependence: further evidence for second hand exposure hypothesis. J. Addict. Dis. 25(1):15–21 (2006).
C. Rossano, L.F. De Luca, V. Firetto, and F. Fossi. Activity and tolerability of buprenorphine after parenteral and sublingual administration. Clin. Ther. 5(1):61–68 (1982).
D. Dini, T. Fassio, A. Gottlieb, and M. Gini. Controlled study of the analgesic effect and tolerability of buprenorphine in cancer patients. Minerva Med. 77(3–4):93–104 (1986).
A. Cowan, J. W. Lewis, and I. R. Macfarlane. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br. J. Pharmacol. 60:537–545 (1977).
A. Cowan, J. C. Doxey, and E. J. R. Harry. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br. J. Pharmacol. 60:547–554 (1977).
A. Cowan. Update on the general pharmacology of buprenorphine. In A. Cowan, and J. W. Lewis (eds.), Buprnorphine: Combatting Drug Abuse with a Unique Opioid. Wiley-Liss, New York, 1995, pp. 31–47.
K. A. Sporer. Buprenorphine: a primer for emergency physicians. Ann. Emerg. Med. 43(5):580–584 (2004)(Review).
A. Cowan. Buprenorphine: new pharmacological aspects. Int. J. Clin. Pract. Suppl. 133:3–8 (2003).
I. R. Wilding, S. S. Davis, G. H. Rimoy, P. Rubin, T. Kurihar-Bergstrom, V. Tipnis, B. Berner, and J. Nightingale. Pharmacokinetic evaluation of transdermal buprenorphine in man. Int. J. Pharm. 132:81–87 (1996).
H. C. Evans, and S. E. Easthope. Transdermal buprenorphine. Drugs 63(19):1999–2010 (2003).
R. Sittl. Transdermal buprenorphine in the treatment of chronic pain. Expert Rev. Neurother. 5(3):315–323 (2005).
J. Sorge, and R. Sittl. Transdermal buprenorphine in the treatment of chronic pain: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. Clin. Ther. 26(11):1808–1820 (2004).
K. Budd. Buprenorphine and the transdermal system: the ideal match in pain management. Int. J. Clin. Pract. Suppl. 133:9–14 (2003).
L. Radbruch. Buprenorphine TDS: the clinical development-rationale and results. Int. J. Clin. Pract. Suppl. 133:15–18 (2003).
R. E. Johnson, P. J. Fudala, and R. Payne. Buprenorphine: considerations for pain management. J. Pain Symptom Manage 29(3):297–326 (2005)(Review).
H. C. Evans, and S. E. Easthope. Transdermal buprenorphine. Drugs 63(19):1999–2010 (2003).
KFDA. Test Guidelines for Safety Evaluation of Drugs. KFDA Notification. No. 1999-61 (1999).
J. V. Roughan, and P. A. Flecknell. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating post-operative pain in animals. Lab. Animals 36(3):322–343 (2002).
C. G. Pick, Y. Peter, S. Schreiber, and R. Weizman. Pharmacological characterization of buprenorphine, a mixed agonist-antagonist with κ3 analgesia. Brain Res. 744:41–46 (1997).
W. A. Ritschel, and G. L. Kearns. Volume of distribution and distribution coefficient. In: Handbook of Basic Pharmacokinetics: Concepts and Applications, American Pharmaceutical Association, Washington DC, pp 178–188.
P. A. Flecknell, and J. H. Liles. Assessment of the analgesic action of opioid agonist–antagonists in the rabbit. J. Ass. Vet. Anaesth. 17:24–29 (1990).
A. Polettini, and M. A. Huestis. Simultaneous determination of buprenorphine, norbuprenorphine and buprenorphine-glucuronide in plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 754:447–459 (2001).
S. Gopal, T. B. Tzeng, and A. Cowan. Development and validation of a sensitive analytical method for the simultaneous determination of buprenorphine and norbuprenorphine in human plasma. Eur. J. Pharmacol. 51:147–151 (2001).
M. Ohtani, H. Kotaki, K. Nishitateno, Y. Sawada, and T. Iga. Pharmacokinetic analysis of enterohepatic circulation of buprenorphine and its active metabolite, norbuprenorphine, in rats. Drug Metab. Dispos. 22:2–7 (1985).
A. Ceccato, R. Klinkenberg, P. Hubert, and B. Streel. Sensitive determination of buprenorphine and its N-dealkylated metabolite norbuprenorphine in human plasma by liquid chromatography coupled to tandem mass spectrometry. J. Pharm. Biomed. Anal. 32:619–631 (2003).
D. E. Moody, M. H. Slawson, E. C. Strain, J. D. Laycock, A. C. Spanbauer, and R. L. Foltz. A liquid chromatographic-electrospray ionization-tandem mass spectrometric method for determination of buprenorphine, its metabolite, norbuprenorphine and a coformulant, Naloxone, that is suitable for in vivo and in vitro metabolism. Anal. Biochem. 306:31–39 (2002).
G. P. Hernandez-Delgadillo, and S. L. Cruz. Endogenous opioids are involved in morphine and dipyrone analgesic potentiation in the tail flick test in rats. Eur. J. Pharmacol. 546(1–3):54–59 (2006).
H. O. J. Collier, L. C. Dinneen, A. Chistine, A. Johnson, and C. Schneider. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. Chemother. 32:295–310 (1968).
J. H. Draize, G. Woodand, and H. O. Calvery. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 82:377–390 (1994).
R. Sittl. Buprenorphine transdermal patch: clinical expert report. Germany: Grunenthat GmbH 2000/Hannah C. Evans & Stephanie E. Easthope 2003, Transdermal Buprenorphine. Drug 63(19):1999–2010 (2003).
R. L. Bronaugh, and R. F. Stewart. Methods for in vitro percutaneous absorption studies V: Permeation through damaged skin. J. Pharm. Sci. 74(10):1062–1066 (1985).
R. L. Bronaugh, and R. F. Stewart. Methods for in vitro percutaneous absorption studies IV: the flow-through diffusion cell. J. Pharm. Sci. 74:64–67 (1985).
A. M. Kligman. A biological brief on percutaneous absorption. Drug Dev. Ind. Pharm. 9(4):521–560 (1983).
P. Sartorelli, H. R. Andersen, J. Angerer, J. Corish, H. Drexler, T. Göen, P. Griffin, S. A. M. Hotchkiss, F. Larese, L. Montomoli, J. Perkins, M. Schmelz, J. van de Sandt, and F. Williams. Percutaneous penetration studies for risk assessment. Environ. Toxicol. Pharmacol. 8(2):133–152 (2000).
ECETOC (1993) In: Percutaneous Absorption, European Centre for Ecotoxicology and Toxicology of Chemicals, Brussels, Monograph Nr. 20, 1–80.
W. R Good, M. S. Powers, P. Campbell, and L. Schenkel. A new transdermal delivery system for estradiol. J. Control. Release 2:89–97 (1985).
N. L. Benowitz, K. Chan, C. P. Denaro, and P. Jacob III. Stable isotope method for studying transdermal drug absorption: the nicotine patch. Clin. Pharmacol. Ther. 50:286–293 (1991).
M. Ohtani, H. Kotaki, Y. Sawada, and T. Iga. Comparative analysis of buprenorphine- and norbuprenorphine-induced analgesic effects based on pharmacokinetic-pharmacodynamic modeling. J. Pharmacol. Exp. Ther. 272:505–510 (1995).
A. Yassen, E. Olofsen, A. Dahan, and M. Danhof. Pharmacokinetic-pharmacodynamic modeling of the antinociceptive effect of buprenorphine and fentabyl in rats: role of receptor equilibration kinetics. J. Pharmacol. Exp. Ther. 313:1136–1149 (2005).
R. Kawai, R.E. Carson, B. Dunn, A. H. Newman, K. C. Rice, and R. G. Blasberg. Regional brain measurement of Bmax and KD with the opiate antagonist cyclofoxy: equilibrium studies in the conscious rat. J. Cereb. Blood Flow Metab. 11(4):529–544 (1991).
H. Kusuhara, and Y. Sugiyama. Efflux transport systems for drugs at the blood–brain barrier and blood–cerebrospinal fluid barrier (Part 1). Drug Discov. Today 6:150–156 (2001).
T. K. Henthorn, Y. Liu, M. Mahapatro, and K. Y. Ng. Active transport of fentanyl by the blood–brain barrier. J. Pharmacol. Exp. Ther. 289:1084–1089 (1999).
S. J. Thompson, K. Koszdin, and C. M. Bernards. Opiate-induced analgesia is increased and prolonged in mice lacking P-glycoprotein. Anesthesiology 92:1392–1399 (2000).
M. Rodriguez, I. Ortega, I. Soengas, E. Suarez, J. C. Lukas, and R. Calvo. Effect of P-glycoprotein inhibition on methadone analgesia and brain distribution in the rat. J. Pharm. Pharmacol. 56:367–374 (2004).
T. Suzuki, C. Zaima, Y. Moriki, T. Fukami, and K. Tomono. P-glycoprotein mediates brain-to-blood efflux transport of buprenorphine across the blood–brain barrier. J. Drug Target. 15(1):67–74 (2007).
J. W. Wiechers. The barrier function of the skin in relation to percutaneous absorption of drugs. Pharm. Weekbl. 11:185–198 (1989).
R. C. Wester, and P. K. Noonan. Relevance of animal models for percutaneous absorption. Int. J. Pharm. 7:99–110 (1980).
R. Panchagnula, K. Stemmer, and W. A. Ritschel. Animal models for transdermal drug delivery. Methods Find. Exp. Clin. Pharmacol. 19(5):335–341 (1997).
S. L. Borgland. Acute opioid receptor desensitization and tolerance: is there a link? Clin. Exp. Pharmacol. Physiol. 28:147–154 (2001).
D. Debruyne, T. Quentin, G. Poisnel, V. Lelong-Boulouard, L. Barre, and A. Coquerel. Acute and chronic administration of clorazepate modifies the cell surface regulation of μ-opioid receptors induced by buprenorphine in specific regions of the rat brain. Brain Res. 1052:222–231 (2005).
A. Cowan. Buprenorphine: new pharmacological aspects. Int. J. Clin. Pract. Suppl 133:3–8 (2003).
T. Christoph, B. Kogel, K. Schiene, M. Meen, J. De Vry, and E. Friderichs. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur. J. Pharmacol 507:87–98 (2005).
L. Grumbach. The prediction of analgesic activity in man by animal testing. In: R. S. Knighton, P. R. Dumke (eds.), Pain, 15th International Symposium, Detroit, 1964. Little Brown, Boston, 1996, pp. 163–182.
M. Eaton. Common animal models for spasticity and pain. J. Rehabil. Res. Dev. 40(4) Supplement:41–54 (2003).
I. Lizasoain. Buprenorphine: bell-shaped dose response curve for its antagonist effects. Gen. Pharmacol. 22 297–300 (1991).
K. Lutfy, S. Eitan, C. D. Bryant, Y. C. Yang, N. Saliminejad, W. Walwyn, B. L. Kieffer, H. Takeshima, and F. I. Carroll. Buprenorphine-induced antinociception is mediated by mu-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J. Neurosci. 23(32):10331–10337 (2003).
I. Korzeniewska-Rybicka, and A. Plaznik. Analgesic effects of antidepressant drugs. Pharmacol. Biochem. Behav. 59:331–8 (1998).
S. W. Hajare, S. Chandra, S.K. Tandon, J. Sarma, J. Lal, and A. G. Telang. Analgesic and antipyretic activities of Dalbergia Sissoo leaves. Indian J. Pharmacol. 32:357–60 (2000).
K. D. Effraim, U. A. Osunkwo, P. Onyeyilli, and A. Ngulde. Preliminary investigation of possible antinociceptive activity of aqueous leaf extract Ziziphus spina Christi (Linn). Indian J. Pharmacol. 30:271–272 (1998).
Acknowledgements
We would like to thank Dr. Chad Brown for his help in preparation of this manuscript and Mr. Jae Hee Jang for his skillful technical support. The authors acknowledge Yun Jeong Kim and Seung Jin Baek of Biotoxtech, Korea, for conducting the skin irritation and analgesic experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, I., Kim, D., Song, J. et al. Buprederm™, a New Transdermal Delivery System of Buprenorphine: Pharmacokinetic, Efficacy and Skin Irritancy Studies. Pharm Res 25, 1052–1062 (2008). https://doi.org/10.1007/s11095-007-9470-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9470-6