Abstract
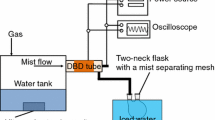
A novel dielectric-barrier-discharge (DBD) loop reactor was designed for the efficient degradation of cyanide anion (CN−) in water. The circulation of cyanide water as a falling film through plasma gas discharge zone enhanced gas–liquid mass and energy transfer and induced formation of H2O2 which was associated with the efficient destruction of CN−. It was observed that among different discharge gases, the CN− degradation rate decreased in the order of Ar > air > H2/air mixture. Depending on discharge voltage, the treatment time for complete removal of 100 ppm CN− in this DBD loop reactor is in the range 120–300 min. The dose of Cu2+ catalyst in combination with in situ production of H2O2 enhanced the destruction of CN− apparently in this DBD loop reactor. The treatment time for complete degradation of 100 ppm CN− decreased from 180 min with Ar DBD discharge alone to 40 min with 40 mg/L dose of Cu2+ ion in water, making it an efficient means to degrade cyanide water.









Similar content being viewed by others
References
Dasha RR, Gaur A, Balomajumderb C (2009) Cyanide in industrial wastewaters and its removal: a review on biotreatment. J Hazard Mater 163:1–11
Dasha RR, Balomajumderb C, Kumarc A (2009) Removal of cyanide from water and wastewater using granular activated carbon. Chem Eng J 146:408–413
Teixeira LAC, Andia JPM, Yokoyama L, Araujo FVF, Sarmiento CM (2013) Oxidation of cyanide in effluents by Caro’s Acid. Miner Eng 45:81–87
Kitis M, Akcil A, Karakaya E, Yigit NO (2005) Destruction of cyanide by hydrogen peroxide in tailings slurries from low bearing sulphidic gold ores. Miner Eng 18:352–362
Zwikl JR, Buchko NS, Junkins DR (1982) Physical/chemical treatment of coke plant wastewaters. Environ Prog 1:244–251
Sato K, Aoki M, Noyori R (1998) A “green” route to adipic acid: direct oxidation of cyclohexenes with 30% hydrogen peroxide. Science 281:1646–1647
Gates DD, Siegrist RL (1995) In-situ chemical oxidation of trichloroethylene using hydrogen peroxide. J Environ Eng 121:639–644
Hancu D, Green J, Beckman EJ (2002) H2O2 in CO2: sustainable production and green reactions. Acc Chem Res 35:757–764
Morinaga K (1962) The reaction of hydrogen and oxygen through a silent electric discharge. I. The formation of hydrogen peroxide. Bull Chem Soc Jpn 35:345–348
Morinaga K (1962) The reaction of hydrogen and oxygen through a silent electric discharge. II. The effect of packings on a peroxide formation. Bull Chem Soc Jpn 35:625–626
Zhao JL, Zhou JC, Guo HC, Wang XS, Gong WM (2009) Scale-up synthesis of hydrogen peroxide from H2/O2 with multiple parallel DBD tubes. Plasma Sci Technol 11:181–186
Chandana L, Reddy PM, Subrahmanyam C (2015) Atmospheric pressure non-thermal plasma jet for the degradation of methylene blue in aqueous medium. Chem Eng J 282:116
Banaschik R, Lukes P, Jablonowski H, Hammer MU, Weltmann KD, Kolb JF (2015) Potential of pulsed corona discharges generated in water for the degradation of persistent pharmaceutical residues. Water Res 84:127–135
Valsero MH, Molina R, Schikora H, Müller M, Bayona JM (2013) Removal of cyanide from water by means of plasma discharge technology. Water Res 47:1701–1707
Fortin L, Soucy G, Kasireddy VK, Bernier JL (2000) Novel reactor for cyanide solution treatment. Can J Chem Eng 78:643–649
Jamroz P, Gręda K, Pohl P, Zyrnicki W (2014) Atmospheric pressure glow discharges generated in contact with flowing liquid cathode: production of active species and application in wastewater purification processes. Plasma Chem Plasma Process 34:25–37
Sarla M, Pandit M, Tyagi DK, Kapoor JC (2004) Oxidation of cyanide in aqueous solution by chemical and photochemical process. J Hazard Mater 116:49–56
Acknowledgement
The support of this work by the Fundamental Research Funds for the Central Universities (Grant No. 24720094028) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, B. A Novel Dielectric-Barrier-Discharge Loop Reactor for Cyanide Water Treatment. Plasma Chem Plasma Process 37, 1121–1131 (2017). https://doi.org/10.1007/s11090-017-9791-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-017-9791-0