Abstract
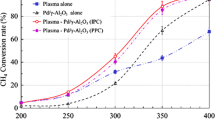
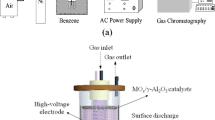
Non-oxidative methane activation over particle-size adjusted alumina catalysts loaded with metal oxide (Al2O3, MgO/Al2O3, and TiO2/Al2O3) was investigated with a dielectric barrier discharge reactor using 10 % CH4 in Ar at plasma induced temperature. Plasma-assisted catalytic activity for direct conversion of methane over the catalysts was compared with that using plasma only. Catalyst hybrid reaction in a non-thermal discharge showed that MgO/Al2O3 had the highest activity for methane conversion. C2, C3, and C4 hydrocarbons were formed as products; ethane, ethylene, and acetylene were predominant over all catalysts. The effect of varying particle size of the MgO/Al2O3 catalyst was also examined. The conversion of methane over MgO/Al2O3 dramatically increased with decreasing catalyst particle size from 1.70 to 0.25 mm. It is interesting to note that distribution of C2 hydrocarbons was tuned by changing the particle size of the catalyst. It was also observed that the gas flow rate, frequency, and power supplied affected direct conversion of methane and selectivity of products significantly.











Similar content being viewed by others
References
Brook EJ, Harder S, Severinghaus J, Steig EJ, Sucher CM (2000) Global Biogeochem Cycles 14:559–571
Lashof DA, Ahuja DR (1990) Nature 344:529–531
Lunsford JH (2000) Catal Today 63:165–174
Ross JRH (1975) In: Roberts MW, Thomas JM (eds) Surface and defect properties of solids, vol 4. The Chemical Society, London
Holmen A (2009) Catal Today 142:2–8
Rostrup-Nielsen JR (1993) Catal Today 18:305–324
Chou L, Cai Y, Zhang B, Niu J, Ji S, Li S (2002) J Nat Gas Chem 11:131–136
Pak S, Lunsford JH (1998) Appl Catal A 168:131–136
Kogelschatz U (2003) Plasma Chem Plasma Process 23:1–46
Kado S, Sekine Y, Fujimoto K (1999) Chem Commun 24:2485–2486
Alvarez-Galvan MC, Mota N, Ojeda M, Rojas S, Navarro RM, Fierro JLG (2011) Catal Today 171:15–23
Młotek M, Sentek J, Krawczyk K, Schmidt-Szałowski K (2009) Appl Catal A Gen 366:232–241
Liu C-J, Mallinson R, Lobban L (1998) J Catal 179:326–334
Wang Z-J, Zhao Y, Cui L, Du H, Yao P, Liu C-J (2007) Green Chem 9:554–559
Kim SH, Jung C-H, Sahu N, Park D, Yun JY, Ha H, Park JY (2013) Appl Catal A Gen 454:53–58
Istadi I, Amin NAS (2006) Fuel 85:577–592
Xu Y, Bao X, Lin L (1998) J Catal 216:386–395
Kim S-S, Lee H, Song HK, Na B-K (2006) J Ind Eng Chem 12:558–565
Arif M, Malik SA, Xuaen-Zhen J (1998) J Gas Chem 2:166
Sentek J, Krawczyk K, Młotek M, Kalczewska M, Kroker T, Kolb T, Schenk A, Gericke K-H, Schmidt-Szałowski K (2010) Appl Catal B Environ 94:19–26
Tu X, Whitehead JC (2012) Appl Catal B Environ 125:439–448
Jo S, Lee DH, Kang SW, Song HY (2013) Phys Plasma 20:123507–123514
Gallon HJ, Tu X, Whitehead JC (2012) Plasma Process Polym 9:90–97
Tu X, Gallon JH, Whitehead JC (2011) J Phys D Appl Phys 44:482003–482007
Tu X, Gallon HJ, Twigg MV, Gorry PA, Whitehead JC (2011) J Phys D Appl Phys 44:274007–274011
Wan JKS, Chen YG, Lee YJ, Derew MC (2000) Res Chem Intermed 26:599–619
Nozaki T, Hattori A, Okazaki K (2004) Catal Today 98:607–616
Kostov KG, Honda RY, Alves LMS, Kayama ME (2009) Braz J Phys 39:322–325
Manley TC (1943) Trans Electrochem Soc 84:83–96
Nozaki T, Tsukijihara H, Fukui W, Okazki K (2007) Energy Fuels 21:2525–2530
Coogan JJ, Sappey AD (1996) IEEE Trans Plasma Sci 24:91–92
Yang Y (2003) Plasma Chem Plasma Process 23:327–346
Hammer T, Kappes T, Baldauf M (2004) Catal Today 89:5–14
Robertsona J (2004) Eur Phys J Appl Phys 28:265–291
Chen HL, Lee HM, Chen SH, Chang MB, Yu SJ, Li SN (2009) Environ Sci Technol 43:2216–2227
Trionfetti C, Agıral A, Gardeniers JGE, Lefferts L, Seshan K (2008) J Phys Chem C 112:4267–4274
Indarto A, Choi JW, Lee H, Song HK (2006) J Nat Gas Chem 15:87–92
Riccardi C, Barni R, Fontanesi M, Tosi P (2000) Chem Phys Lett 329:66–70
Indarto A, Choi JW, Lee H, Song HK (2005) J Nat Gas Chem 14:13–21
Lieberman MA, Lichtenberg AJ (1994) Principles of plasma discharges and material processing, 1st edn. Wiley, New York
Lee DH, Kim K-T, Song Y-H, Kang WS, Jo S (2013) Plasma Chem Plasma Process 33:249–269
Acknowledgments
The authors acknowledge the financial support received from the Korea Research Council for Industrial Science and Technology (ISTK) of the Republic of Korea, Grant Number B551179-11-03-00.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kasinathan, P., Park, S., Choi, W.C. et al. Plasma-Enhanced Methane Direct Conversion over Particle-Size Adjusted MOx/Al2O3 (M = Ti and Mg) Catalysts. Plasma Chem Plasma Process 34, 1317–1330 (2014). https://doi.org/10.1007/s11090-014-9574-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-014-9574-9