Abstract
Neural electrical activities are due to the movement of ions in/out of the neuron and can be modulated by an external electric field. Moreover, clinical evidences reveal that the modulated activities of brain tissue by an external electric field are associated with normal or pathological brain functions. In this paper, we investigated the spatiotemporal activities of a network of neurons considering an AC electric field. It is shown that the external electric field has a significant impact on the activities of the neural network. The strong external electric field facilitates the neuron firing action potentials and enhances the mean firing rate of the network, but disrupts the synchronicity of the activities of the neural network. The information entropy revealed that the external field is capable of changing the amount of information in the neural network and the interspike internals distribution can also be changed by the external field regardless the network parameters. It is observed a v-shape resonant area in the \(E_\mathrm{appl}\)-f (field intensity–field frequency) parameter space, where the neural network exhibits a high firing rate but weak synchronicity and low value of information entropy. Moreover, the effect of the electric field on the spatiotemporal activities of the neural network is detected in different connection fraction and network size. Our current work gives the insight into the effect of the external electric field on the spatiotemporal activities of the neural network.









Similar content being viewed by others
References
Ahlfors, S.P., Simpson, G.V., Dale, A.M., Belliveau, J.W., Liu, A.K., Korvenoja, A., Virtanen, J., Huotilainen, M., Tootell, R.B.H., Aronen, H.J., Ilmoniemi, R.J.: Spatiotemporal activity of a cortical network for processing visual motion revealed by MEG and fMRI. J Neurophysiol. 82, 2545–2555 (1999)
Gerstner, W., Kistler, W.M.: Spiking Neuron Models: Single Neurons, Populations, Plasticity, 1st edn. Cambridge University Press, Cambridge (2002)
Varela, F., Lachaux, J., Rodriguez, E., Martinerie, J.: The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239 (2001)
Roelfsema, P.R., Engel, A., König, P., Singer, W.: Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature 385, 157–161 (1997)
Bressler, S.: Interareal synchronization in the visual cortex. Behav. Brain Res. 76, 37–49 (1996)
Rodriguez, E.: Perceptions shadow: long-distance synchronization of human brain activity. Nature 397, 430–433 (1999)
Foster, D.J., Wilson, M.A.: Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683 (2006)
Melloni, L., Molina, C., Pena, M., Torres, D., Singer, W., Rodriguez, E.: Synchronization of neural activity across cortical areas correlates with conscious perception. J. Neurosci. 27, 2858–2865 (2007)
Riehle, A., Grün, S., Diesmann, M., Aertsen, A.: Spike synchronization and rate modulation differentially involved in motor cortical function. Science 278, 1950–1953 (1997)
Axmacher, N., Mormann, F., Fernández, G., Elger, C.E., Fell, J.: Memory formation by neuronal synchronization. Brain Res. Rev. 52, 170–182 (2006)
Turrigiano, G.G., Nelson, S.B.: Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 5, 97–107 (2004)
Liu, Y., Zhang, L.I., Tao, H.W.: Heterosynaptic scaling of developing GABAergic synapses: dependence on glutamatergic input and developmental stage. J. Neurosci. 27, 5301–5312 (2007)
Ledoux, E., Brunel, N.: Dynamics of networks of excitatory and inhibitory neurons in response to time-dependent inputs. Front. Comput. Neurosci. 5, 25 (2011)
Ma, J., Song, X., Jin, W., Wang, C.: Autapse-induced synchronization in a coupled neuronal network. Chaos Solitons Fractals 80, 31–38 (2015)
Gong, Y., Xie, Y., Lin, X., Hao, Y.: Non-gaussian noise-optimized intracellular cytosolic calcium oscillations. BioSystems. 103, 13–17 (2011)
Zhang, R., Hou, Z., Xin, H.: Effects of non-Gaussian noise near supercritical Hopf bifurcation. Phys. A 390, 147–153 (2011)
Tiesinga, P.H.E., Fellous, J.M., Salinas, E., José, J.V., Sejnowski, T.J.: Synchronization as a mechanism for attentional gain modulation. Neurocomputing 58–60, 641–646 (2004)
Bogaard, A., Parent, J., Zochowski, M., Booth, V.: Interaction of cellular and network mechanisms in spatiotemporal pattern formation in neuronal networks. J. Neurosci. 29, 1677–1687 (2009)
Xu, B., Gong, Y., Wang, L., Wu, Y.: Multiple synchronization transitions due to periodic coupling strength in delayed Newman–Watts networks of chaotic bursting neurons. Nonlinear Dyn. 72, 79–86 (2013)
Bikson, M., Inoue, M., Akiyama, H., Deans, J.K., Fox, J.E., Miyakawa, H., Jefferys, J.G.R.: Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J. Physiol. 557, 175–190 (2004)
Radman, T., Su, Y., An, J.H., Parra, L.C., Bikson, M.: Spike timing amplifies the effect of electric fields on neurons: implications for endogenous field effects. J. Neurosci. 27, 3030–3036 (2007)
McCaig, C.D., Song, B., Rajnicek, A.M.: Electrical dimensions in cell science. J. Cell Sci. 122, 4267–4276 (2009)
Robertson, J.A., Théberge, J., Weller, J., Drost, D.J., Prato, F.S., Thomas, A.W.: Low-frequency pulsed electromagnetic field exposure can alter neuroprocessing in humans. J. R. Soc. Interface. 7, 467–473 (2010)
Dogru, A.G., Tunik, S., Akpolat, V., Dogru, M., Saribas, E.E., Kaya, F.A., Nergiz, Y.: The effects of pulsed and sinusoidal electromagnetic fields on E-cadherin and type IV collagen in gingiva: a histopathological and immunohistochemical study. Adv. Clin. Exp. Med. 22, 245–252 (2013)
Che, Y.Q., Wang, J., Si, W.J., Fei, X.Y.: Phase-locking and chaos in a silent Hodgkin–Huxley neuron exposed to sinusoidal electric field. Chaos Solitons Fractals 39, 454462 (2009)
Yi, G.S., Wang, J., Han, C.X., Deng, B., Wei, X.: Spiking patterns of a minimal neuron to ELF sinusoidal electric field. Appl. Math. Model. 36, 3673–3684 (2012)
Che, Y., Wang, J., Deng, B., Wei, X., Han, C.: Bifurcations in the Hodgkin–Huxley model exposed to DC electric fields. Neurocomputing 81, 41–48 (2012)
Wang, C., Ma, J., Jin, W.Y., Wu, Y.: Electric Field-induced dynamical evolution of spiral wave in the regular networks of Hodgkin–Huxley neurons. Appl. Math. Comput. 218, 4467–4474 (2011)
Stuchly, M.A., Dawson, T.W.: Interaction of low-frequency electric and magnetic fields with the human body. Proc. IEEE 88, 643–664 (2000)
Johansen, C.: Electromagnetic fields and health effects-epidemiologic studies of cancer, diseases of the central nervous system and arrhythmia-related heart disease. Scand. J. Work Environ Health. 30(Suppl 1), 1–30 (2004)
Hodgkin, A.L., Huxley, A.F.: A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. (Lond.) 117, 500–544 (1952)
Shilnikov, A.: Complete dynamical analysis of a neuron model. Nonlinear Dyn. 68, 305–328 (2011)
Balenzuela, P., Garca-Ojalvo, J.: Role of chemical synapses in coupled neurons with noise. Phys. Rev. E. 72, 021901 (2005)
Kotnik, T., Miklavčiě, D., Slivnik, T.: Time course of transmembrane voltage induced by time-varying electric fields a method for theoretical analysis and its application. Bioelectrochem. Bioenerg. 45, 3–16 (1998)
Gianni, M., Liberti, M., Apollonio, F., DInzeo, G.: Modeling electromagnetic fields detectability in a HH-like neuronal system: stochastic resonance and window behavior. Biol. Cybern. 94, 118–127 (2006)
Doruk, R.O.: Control of repetitive firing in Hodgkin–Huxley nerve fibers using electric fields. Chaos Solitons Fractals 52, 66–72 (2013)
Marszalek, P., Liu, D.S., Tsong, T.Y.: Schwan equation and transmembrane potential induced by alternating electric field. Biophys. J. 58, 1053–1058 (1990)
Fink, C.G., Booth, V., Zochowski, M.: Cellularly-driven differences in network synchronization propensity are differentially modulated by firing frequency. PLoS Comput. Biol. 7, e1002062 (2011)
Bullmore, E., Sporns, O.: Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198 (2009)
Stam, C.J.: Modern network science of neurological disorders. Nat. Rev. Neurosci. 10, 186–198 (2014)
Watts, D.J., Strogatz, S.H.: Collective dynamics of small-world networks. Nature 393, 440–442 (1998)
Fox, R.F., Gatland, I.R., Roy, R., Vemuri, G.: Fast, accurate algorithm for numerical simulation of exponentially correlated colored noise. Phys. Rev. A. 38, 5938–5940 (1988)
Abarbanel H.D.I., Tumer, E.C. Reading neural encodings using phase space methods, perspectives and problems in nonlinear science. In: Kaplan E, Marsden JE, Sreenivasan KR (eds) A Celebratory Volume in Honor of Lawrence Sirovich, Springer Applied Mathematical Sciences Series. Springer, Berlin (2003)
Wang, H., Wang, L., Yu, L., Chen, Y.: Response of Morris–Lecar neurons to various stimuli. Phys. Rev. E. 83, 021915 (2011)
Van der Mark, M., Vermeulen, R., Nijssen, P.C.G., Mulleners, W.M., Sas, A.M.G., van Laar, T., Kromhout, H., Huss, A.: Extremely low-frequency magnetic field exposure, electrical shocks and risk of Parkinsons disease. Int. Arch. Occup. Environ. Health. 88, 227–234 (2015)
Li, Y., Mogul, D.J.: Electrical control of epileptic seizures. J. Clin. Neurophysiol. 24, 197–204 (2007)
Acknowledgments
This work was supported by the National Natural Science Foundation of China with Grants No. 11447027, and the Fundamental Research Funds for the Central Universities with No. GK20 1503025.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
1.1 Description of the neuronal model
The neuron in our neural network is chosen as the Hodgkin–Huxley conductance-based model, which describes how action potentials are initiated and propagated in a single neuron. Here, the neuron is treated as a single-patch model, and the current along the axon is ignored. To make the model closely match the real nervous system, the angle (\(\theta \)) between the field line and the exterior normal direction of the patches has a uniform distribution, based on the complex structure neural network and neurons.
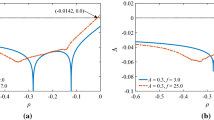
On the other hand, we consider the action of the exogenous electric fields at the cellular level as a membrane voltage perturbation and introducing an additive correction term \(V_E\) for the reversal potential. In fact, the external fields interact with the ions in the neuron and induce a reset of the distribution of ions both inside and outside membranes. Thus, the reversal potential should be modified. Based on the theory of the electric field (induced by ion distribution) force just balances the diffusion force at equilibrium, in the current model, we suppose that force induced by an external electrical field \(V_E\) is balanced by the changes in distribution of ions. Thus, the final reversal potential \(E_\mathrm{ion}^\mathrm{final}\) (with effect of an external field) can be simply written as: \(E_\mathrm{ion}^\mathrm{final}=-V_E+E_\mathrm{ion}\). Here, \(E_\mathrm{ion}\) is the reversal potential without of an external field.
Rights and permissions
About this article
Cite this article
Wang, H., Chen, Y. Spatiotemporal activities of neural network exposed to external electric fields. Nonlinear Dyn 85, 881–891 (2016). https://doi.org/10.1007/s11071-016-2730-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-016-2730-4