Abstract
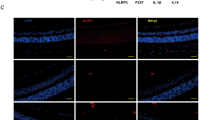
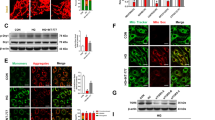
Diabetic retinopathy (DR) is a multifactorial disease characterized by reactive gliosis and disbalance of angiogenesis regulators, contributing to endothelial dysfunction and microvascular complications. This study was organized to elucidate whether poly(ADP-ribose) polymerase-1 (PARP-1) inhibition could attenuate diabetes-induced damage to macroglia and correct angiogenic disbalance in diabetic rat retina. After 8 weeks of streptozotocin (STZ)-induced diabetes, Wistar male rats were treated with PARP-1 inhibitors, nicotinamide (NAm) or 3-aminobenzamide (3-AB) (100 and 30 mg/kg/daily i.p., respectively), for 14 days. After the 10-weeks experiment period, retinas were undergone an immunohistochemical staining for glial fibrillary acidic protein (GFAP), while western blots were performed to evaluate effects of PAPR-1 inhibitors on the levels of PARP-1, poly(ADP-ribosyl)ated proteins (PARs), GFAP, and angiostatin isoforms. Diabetes induced significant up-regulation and activation of retinal PARP-1, reactive gliosis development, and GFAP overexpression compared to non-diabetic control. Moreover, extensive fragmentation of both PARP-1 and GFAP (hallmarks of apoptosis and macroglia reactivation, respectively) in diabetic retina was also observed. Levels of angiostatin isoforms were dramatically decreased in diabetic retina, sustaining aberrant pro-angiogenic condition. Both NAm and 3-AB markedly attenuated damage to macroglia, evidenced by down-regulation of PARP-1, PARs and total GFAP compared to diabetic non-treated group. PARP-1-inhibitory therapy prevented formation of PARP-1 and GFAP cleavage-derived products. In retinas of anti-PARP-treated diabetic animals, partial restoration of angiostatin’s levels was shown. Therefore, PARP-1 inhibitors counteract diabetes-induced injuries and manifest retinoprotective effects, including attenuation of reactive gliosis and improvement of angiogenic status, thus, such agents could be considered as promising candidates for DR management.





Similar content being viewed by others
References
Fong DS, Aiello LP, Ferris FL, Klein R (2004) Diabetic retinopathy. Diabetes Care 27(10):2540–2553. doi:10.2337/diacare.27.10.2540
Antonetti DA, Klein R, Gardner TW (2012) Diabetic retinopathy. N Engl J Med 366(13):1227–1239. doi:10.1056/NEJMra1005073
Kern TS (2014) Interrelationships between the retinal neuroglia and vasculature in diabetes. Diabetes Metab J 38(3):163–170. doi:10.4093/dmj.2014.38.3.163
Paquet-Durand F, Silva J, Talukdar T, Johnson LE, Azadi S, van Veen T, Ueffing M, Hauck SM, Ekström PA (2007) Excessive activation of poly(ADP-ribose) polymerase contributes to inherited photoreceptor degeneration in the retinal degeneration 1 mouse. J Neurosci 27(38):10311–10319. doi:10.1523/JNEUROSCI.1514-07.2007
Chaitanya GV, Alexander JS, Babu PP (2010) PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal 8:31. doi:10.1186/1478-811X-8-31
Baydas G, Tuzcu M, Yasar A, Baydas B (2004) Early changes in glial reactivity and lipid peroxidation in diabetic rat retina: effects of melatonin. Acta Diabetol 41(3):123–128
Coorey NJ, Shen W, Chung SH, Zhu L, Gillies MC (2012) The role of glia in retinal vascular disease. Clin Exp Optom 95(3):266–281. doi:10.1111/j.1444-0938.2012.00741.x
Ly A, Yee P, Vessey KA, Phipps JA, Jobling AI, Fletcher EL (2011) Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest Ophthalmol Vis Sci 52(13):9316–9326. doi:10.1167/iovs.11-7879
Dorrell MI, Aguilar E, Jacobson R, Trauger SA, Friedlander J, Siuzdak G, Friedlander M (2010) Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia 58(1):43–54. doi:10.1002/glia.20900
Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A (2006) Müller cells in the healthy and diseased retina. Prog Retin Eye Res 25(4):397–424
Sugaya-Fukasawa M, Watanabe T, Tamura M, Egashira S, Hisatomi H (2011) Glial fibrillary acidic protein is one of the key factors underlying neuron-like elongation in PC12 cells. Exp Ther Med 2(1):85–87. doi:10.3892/etm.2010.162
Suárez I, Bodega G, Rubio M, García-Segura LM, Fernández B (1994) Astroglial induction of in vivo angiogenesis. J Neural Transplant Plast 5(1):1–10. doi:10.1155/NP.1994.1
Abcouwer SF (2012) Angiogenic factors and cytokines in diabetic retinopathy. J Clin Cell Immunol 1(11):1–12. doi:10.4172/2155-9899
Simó R, Carrasco E, García-Ramírez M, Hernández C (2006) Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev 2(1):71–98
Cao Y, Xue L (2004) Angiostatin. Semin Thromb Hemost 30(1):83–93. doi:10.1055/s-2004-822973
Spranger J, Hammes HP, Preissner KT, Schatz H, Pfeiffer AF (2000) Release of the angiogenesis inhibitor angiostatin in patients with proliferative diabetic retinopathy: association with retinal photocoagulation. Diabetologia 43(11):1404–1407. doi:10.1007/s001250051546
Coppini LP, Visniauskas B, Costa EF, Filho MN, Rodrigues EB, Chagas JR, Farah ME, Barros NM, Carmona AK (2015) Corneal angiogenesis modulation by cysteine cathepsins: in vitro and in vivo studies. Exp Eye Res 134:39–46. doi:10.1016/j.exer.2015.03.012
Drixler TA, Borel Rinkes IH, Ritchie ED, Treffers FW, van Vroonhoven TJ, Gebbink MF, Voest EE (2001) Angiostatin inhibits pathological but not physiological retinal angiogenesis. Invest Ophthalmol Vis Sci 42(13):3325–3330
Shyong MP, Lee FL, Kuo PC, Wu AC, Cheng HC, Chen SL, Tung TH, Tsao YP. Reduction of experimental diabetic vascular leakage by delivery of angiostatin with a recombinant adeno-associated virus vector. Mol Vis 13:133–141
Kim J, Kim Y, Kim J, Kang T (2014) PARP1 activation/expression modulates regional-specific neuronal and glial responses to seizure in a hemodynamic-independent manner. Cell Death Dis 5:e1362. doi:10.1038/cddis.2014.331
Obrosova IG, Minchenko AG, Frank RN, Seigel GM, Zsengeller Z, Pacher P, Stevens MJ, Szabó C (2004) Poly(ADP-ribose) polymerase inhibitors counteract diabetes- and hypoxia-induced retinal vascular endothelial growth factor overexpression. Int J Mol Med 14(1):55–64
Kuchmerovska T, Shymanskyy I, Donchenko G, Kuchmerovskyy M, Pakirbaieva L, Klimenko A (2004) Poly(ADP-ribosyl)ation enhancement in brain cell nuclei is associated with diabetic neuropathy. J Diabetes Complicat 18(4):198–204. doi:10.1016/S1056-8727(03)00039-4
Chiu J, Xu B, Chen S, Feng B, Chakrabarti S (2008) Oxidative stress-induced, poly(ADP-ribose) polymerase-dependent upregulation of ET-1 expression in chronic diabetic complications. Can J Physiol Pharmacol 86(6):365–372. doi:10.1139/Y08-033
Maiese K, Chong ZZ, Hou J, Shang YC (2009) The vitamin nicotinamide: translating nutrition into clinical care. Molecules 14(9):3446–3485. doi:10.3390/molecules14093446
Stoscheck CM (1990) Quantitation of protein. Methods Enzymol 182:50–68
Tykhomyrov AA, Yusova EI, Diordieva SI, Corsa VV, Grinenko TV (2013) Production and characteristics of antibodies against K1-3 fragment of human plasminogen. Biotechnol Acta 6(1):86–96
Tura A, Schuettauf F, Monnier PP, Bartz-Schmidt KU, Henke-Fahle S (2009) Efficacy of Rho-kinase inhibition in promoting cell survival and reducing reactive gliosis in the rodent retina. Invest Ophthalmol Vis Sci 50(1):452–461. doi:10.1167/iovs.08-1973
Rajesh M, Mukhopadhyay P, Bátkai S, Godlewski G, Haskó G, Liaudet L, Pacher P (2006) Pharmacological inhibition of poly(ADP-ribose) polymerase inhibits angiogenesis. Biochem Biophys Res Commun 350(2):352–357. doi:10.1016/j.bbrc.2006.09.049
Tsacopoulos M, Poitry-Yamate CL, MacLeish PR, Poitry S (1998) Trafficking of molecules and metabolic signals in the retina. Prog Retin Eye Res 17(3):429–442
Rungger-Brändle E, Dosso AA, Leuenberger PM (2000) Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci 41(7):1971–1980
Chen MH, Hagemann TL, Quinlan RA, Messing A, Perng MD (2013) Caspase cleavage of GFAP produces an assembly-compromised proteolytic fragment that promotes filament aggregation. ASN Neuro 5(5):e00125. doi:10.1042/AN20130032
Mouser PE, Head E, Ha KH, Rohn TT (2006) Caspase-mediated cleavage of glial fibrillary acidic protein within degenerating astrocytes of the Alzheimer’s disease brain. Am J Pathol 168(3):936–946
Zhang X, Barile G, Chang S, Hays A, Pachydaki S, Schiff W, Sparrow J (2005) Apoptosis and cell proliferation in proliferative retinal disorders: PCNA, Ki-67, caspase-3, and PARP expression. Curr Eye Res 30(5):395–403. doi:10.1080/02713680590956306
Barot M, Gokulgandhi MR, Patel S, Mitra AK (2013) Microvascular complications and diabetic retinopathy: recent advances and future implications. Future Med Chem 5(3):301–314. doi:10.4155/fmc.12.206
O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Cao Y, Moses M, Lane WS, Sage EH, Folkman J (1994) Angiostatin: a circulating endothelial cell inhibitor that suppresses angiogenesis and tumor growth. Cold Spring Harb Symp Quant Biol 59:471–482
Tykhomyrov AA, Shram SI, Grinenko TV (2015) Role of angiostatins in diabetic complications. Biomed Khim 61(1):41–56. doi:10.1134/S1990750814020140
Pearce JW, Janardhan KS, Caldwell S, Singh B (2007) Angiostatin and integrin αvβ3 in the feline, bovine, canine, equine, porcine and murine retina and cornea. Vet Ophthalmol 10(5):313–319
Cao Y, Chen A, An SS, Ji RW, Davidson D, Llinás M (1997) Kringle 5 of plasminogen is a novel inhibitor of endothelial cell growth. J Biol Chem 272(36):22924–22928
Zhang SX, Sima J, Shao C, Fant J, Chen Y, Rohrer B, Gao G, Ma JX (2004) Plasminogen kringle 5 reduces vascular leakage in the retina in rat models of oxygen-induced retinopathy and diabetes. Diabetologia 47(1):124–131. doi:10.1007/s00125-003-1276-4
Ma J, Li C, Shao C, Gao G, Yang X (2012) Decreased K5 receptor expression in the retina, a potential pathogenic mechanism for diabetic retinopathy. Mol Vis 18:330–336
Sima J, Zhang SX, Shao C, Fant J, Ma J (2004) The effect of angiostatin on vascular leakage and VEGF expression in rat retina. FEBS Lett 564(1–2):19–23. doi:10.1016/S0014-5793(04)00297-2
Li N, Chen J (2014) ADP-ribosylation: activation, recognition, and removal. Mol Cells 37(1):9–16. doi:10.14348/molcells.2014.2245
Drel VR, Pacher P, Stavniichuk R, Xu W, Zhang J, Kuchmerovska TM, Slusher B, Obrosova IG (2011) Poly(ADP-ribose)polymerase inhibition counteracts renal hypertrophy and multiple manifestations of peripheral neuropathy in diabetic Akita mice. Int J Mol Med 28(4):629–635. doi:10.3892/ijmm.2011.709
Virág L, Szabó C (2002) The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 54(3):375–429
Shen CC, Huang HM, Ou HC, Chen HL, Chen WC, Jeng KC (2004) Protective effect of nicotinamide on neuronal cells under oxygen and glucose deprivation and hypoxia/reoxygenation. J Biomed Sci 11(4):472–481. doi:10.1159/000077897
Bai S, Sheline CT (2013) NAD(+) maintenance attenuates light induced photoreceptor degeneration. Exp Eye Res 108:76–83. doi:10.1016/j.exer.2012.12.007
Klaidman L, Morales M, Kem S, Yang J, Chang ML, Adams JD (2003) Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology 69(3):150–157
Kuchmerovska T, Shymanskyy I, Chlopicki S, Klimenko A (2010) 1-methylnicotinamide (MNA) in prevention of diabetes-associated brain disorders. Neurochem Int 56(2):221–228. doi:10.1016/j.neuint.2009.10.004
Miki K, Uehara N, Shikata N, Matsumura M, Tsubura A (2007) Poly(ADP-ribose) polymerase inhibitor 3-aminobenzamide rescues N-methyl-N-nitrosourea-induced photoreceptor cell apoptosis in Sprague-Dawley rats through preservation of nuclear factor-kappaB activity. Exp Eye Res 84(2):285–292. doi:10.1016/j.exer.2006.09.023
Ji D, Li GY, Osborne NN (2008) Nicotinamide attenuates retinal ischemia and light insults to neurones. Neurochem Int 52(4–5):786–798. doi:10.1016/j.neuint.2007.09.012
Acknowledgments
The authors are thankful to Kuznetsov K.I. (PhD, research fellow) and Professor Veselovsky N.S. (Bogomoletz Institute of Physiology of NAS of Ukraine) for their cooperation, expert technical assistance, and fruitful discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no conflicts of interest regarding the publishing of this paper.
Ethical Approval
All applicable international, national, and Institutional Animal Care and Use Committee at Palladin Institute of Biochemistry of NASU (Kyiv, Ukraine; No. 0112U002625) guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Guzyk, M.M., Tykhomyrov, A.A., Nedzvetsky, V.S. et al. Poly(ADP-Ribose) Polymerase-1 (PARP-1) Inhibitors Reduce Reactive Gliosis and Improve Angiostatin Levels in Retina of Diabetic Rats. Neurochem Res 41, 2526–2537 (2016). https://doi.org/10.1007/s11064-016-1964-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1964-3