Abstract
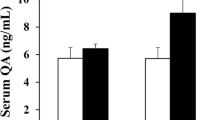
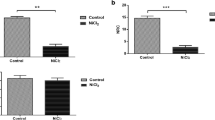
Cuprizone is a copper chelating agent able to selectively damage the white matter in the mouse brain. Recent studies have reported behavioral abnormalities relevant to some of schizophrenia symptoms. While associating white matter damage to the behavioral abnormalities, these previous studies did not rule out the possible impairment in neuronal functions in cuprizone-exposed mice. The aim of this study was to examine brain metabolites of the cuprizone-exposed mice by proton magnetic resonance spectroscopy (1H-MRS). The examined brain regions were the caudoputamen, midbrain, and thalamus; these subcortical regions showed different susceptibilities to cuprizone in terms of demyelination and oligodendrocyte loss in previous studies. Young C57BL/6 mice were fed a standard rodent chow without or with cuprizone (0.2 %) for 6 weeks. At the end, open-field and Y-maze tests were performed to measure the emotional and cognitive behaviors of the animals, followed by 1H-MRS procedure to evaluate the brain metabolites. Cuprizone-exposure increased anxiety levels and impaired spatial working memory. The same treatment increased T2 signal intensity in the cerebral cortex, hippocampus, and caudoputamen, but not in the thalamus. Cuprizone-exposure decreased the concentrations of NAA and NAA+NAAG in caudoputamen, but not in thalamus and midbrain. It decreased levels of Cr+PCr, GPC+PCh and myo-inositol in all the three brain regions. These results provided neurochemical evidence for the impairment in neuronal functions by cuprizone treatment.





Similar content being viewed by others
References
Kesterson JW, Carlton WW (1970) Aqueductal stenosis as the cause of hydrocephalus in mice fed the substituted hydrazine, cuprizone. Exp Mol Pathol 13:281–294
Blakemore WF (1972) Observations on oligodendrocyte degeneration, the resolution of status spongiosus and remyelination in cuprizone intoxication in mice. J Neurocytol 1:413–426
Kesterson JW, Carlton WW (1971) Monoamine oxidase inhibition and the activity of other oxidative enzymes in the brains of mice fed cuprizone. Toxicol Appl Pharmacol 20:386–395
Hoppel CL, Tandler B (1973) Biochemical effects of cuprizone on mouse liver and heart mitochondria. Biochem Pharmacol 22:2311–2318
Venturini G (1973) Enzymic activities and sodium, potassium and copper concentrations in mouse brain and liver after cuprizone treatment in vivo. J Neurochem 21:1147–1151
Matsushima GK, Morell P (2001) The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol 11:107–116
Liebetanz D, Merkler D (2006) Effects of commissural de-and remyelination on motor skill behaviour in the cuprizone mouse model of multiple sclerosis. Exp Neurol 202:217–224
Franco-Pons N, Torrente M, Colomina MT, Vilella E (2007) Behavioral deficits in the cuprizone-induced murine model of demyelination/remyelination. Toxicol Lett 169:205–213
Xiao L, Xu H, Zhang Y, Wei Z, He J, Jiang W, Li X, Dyck L, Devon R, Deng Y (2008) Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol Psychiatry 13:697–708
Zhang Y, Xu H, Jiang W, Xiao L, Yan B, He J, Wang Y, Bi X, Li X, Kong J (2008) Quetiapine alleviates the cuprizone-induced white matter pathology in the brain of C57BL/6 mouse. Schizophr Res 106:182–191
Makinodan M, Yamauchi T, Tatsumi K, Okuda H, Takeda T, Kiuchi K, Sadamatsu M, Wanaka A, Kishimoto T (2009) Demyelination in the juvenile period, but not in adulthood, leads to long-lasting cognitive impairment and deficient social interaction in mice. Prog Neuropsychopharmacol Biol Psychiatry 33:978–985
Xu H, Yang HJ, Zhang Y, Clough R, Browning R, Li XM (2009) Behavioral and neurobiological changes in C57BL/6 mice exposed to cuprizone. Behav Neurosci 123:418–429
Gregg JR, Herring NR, Naydenov AV, Hanlin RP, Konradi C (2009) Downregulation of oligodendrocyte transcripts is associated with impaired prefrontal cortex function in rats. Schizophr Res 113:277–287
Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V (2003) White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry 60:443–456
Port JD, Agarwal N (2011) MR spectroscopy in schizophrenia. J Magn Reson Imaging 34:1251–1261
Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE (2013) Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophr Bull 39:120–129
Fernando K, McLean M, Chard D, MacManus D, Dalton C, Miszkiel KA, Gordon RM, Plant GT, Thompson AJ, Miller DH (2004) Elevated white matter myo-inositol in clinically isolated syndromes suggestive of multiple sclerosis. Brain 127:1361–1369
Narayana PA (2005) Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. J Neuroimaging 15:46S–57S
Aboul-Enein F, Krššák M, Höftberger R, Prayer D, Kristoferitsch W (2010) Reduced NAA-levels in the NAWM of patients with MS is a feature of progression. A study with quantitative magnetic resonance spectroscopy at 3 Tesla. PLoS One 5:e11625. doi:10.1371/journal.pone.0011625
Tezuka T, Tamura M, Kondo MA, Sakaue M, Okada K, Takemoto K, Fukunari A, Miwa K, Ohzeki H, Kano S, Yasumatsu H, Sawa A, Kajii Y (2013) Cuprizone short-term exposure: astrocytic IL-6 activation and behavioral changes relevant to psychosis. Neurobiol Dis 59:63–68
Xuan Y, Yan G, Peng H, Wu R, Xu H (2014) Concurrent changes in (1)H MRS metabolites and antioxidant enzymes in the brain of C57BL/6 mouse short-termly exposed to cuprizone: possible implications for schizophrenia. Neurochem Int 69:20–27
Yang HJ, Wang H, Zhang Y, Xiao L, Clough RW, Browning R, Li XM, Xu H (2009) Region-specific susceptibilities to cuprizone-induced lesions in the mouse forebrain: implications for the pathophysiology of schizophrenia. Brain Res 1270:121–130
Dong HW (2007) Allen reference atlas: a digital color brain atlas of the C57BL/6j male mouse. Wiley, Sesttle
Merkler D, Boretius S, Stadelmann C, Ernsting T, Michaelis T, Frahm J, Brück W (2005) Multicontrast MRI of remyelination in the central nervous system. NMR Biomed 18:395–403
Provencher SW (2001) Automatic quantitation of localizedin vivo1H spectra with LCModel. NMR Biomed 14:260–264
Xu H, Yang HJ, McConomy B, Browning R, Li XM (2010) Behavioral and neurobiological changes in C57BL/6 mouse exposed to cuprizone: effects of antipsychotics. Front Behav Neurosci 4:8. doi:10.3389/fnbeh.2010.00008
Xu H, Yang HJ, Rose GM, Li XM (2011) Recovery of behavioral changes and compromised white matter in C57BL/6 mice exposed to cuprizone: effects of antipsychotic drugs. Front Behav Neurosci 5:31. doi:10.3389/fnbeh.2011.00031
Wang H, Li C, Wang H, Mei F, Liu Z, Shen HY, Xiao L (2013) Cuprizone-induced demyelination in mice: age-related vulnerability and exploratory behavior deficit. Neurosci Bull 29:251–259
Boretius S, Escher A, Dallenga T, Wrzos C, Tammer R, Brück W, Nessler S, Frahm J, Stadelmann C (2012) Assessment of lesion pathology in a new animal model of MS by multiparametric MRI and DTI. NeuroImage 59:2678–2688
Chandran P, Upadhyay J, Markosyan S, Lisowski A, Buck W, Chin CL, Fox G, Luo F, Day M (2012) Magnetic resonance imaging and histological evidence for the blockade of cuprizone-induced demyelination in C57BL/6 mice. Neuroscience 202:446–453. doi:10.1016/j.neuroscience.2011.10.051
Torkildsen Ø, Brunborg LA, Thorsen F, Mørk SJ, Stangel M, Myhr KM, Bø L (2009) Effects of dietary intervention on MRI activity, de- and remyelination in the cuprizone model for demyelination. Exp Neurol 215:160–166
Bardgett ME, Griffith MS, Foltz RF, Hopkins JA, Massie CM, O’Connell SM (2006) The effects of clozapine on delayed spatial alternation deficits in rats with hippocampal damage. Neurobiol Learn Mem 85:86–94
Li Y, Qin HQ, Chen OS, Wang JJ (2004) Behavioral and neurochemical effects of the intrahippocampal co-injection of beta-amyloid protein 1–40 and ibotenic acid in rats. Int J Neurosci 114:1521–1531
Wall PM, Messier C (2001) The hippocampal formation-orbitomedial prefrontal cortex circuit in the attentional control of active memory. Behav Brain Res 127:99–117
Xu H, Yang HJ, Rose GM (2012) Chronic haloperidol-induced spatial memory deficits accompany the upregulation of D1 and D2 receptors in the caudate putamen of C57BL/6 mouse. Life Sci 91:322–328
Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK (2006) Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med 55:302–308
Xie M, Tobin JE, Budde MD, Chen CI, Trinkaus K, Cross AH, McDaniel DP, Song SK, Armstrong RC (2010) Rostrocaudal analysis of corpus callosum demyelination and axon damage across disease stages refines diffusion tensor imaging correlations with pathological teatures. J Neuropathol Exp Neurol 69:704–716
Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM (2007) N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 81:89–131
Friston KJ (1999) Schizophrenia and the disconnection hypothesis. Acta Psychiatr Scand Suppl 99:68–79
Xu H, Li XM (2011) White matter abnormalities and animal models examining a putative role of altered white matter in schizophrenia. Schizophr Res Treatment. doi:10.1155/2011/826976
Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101:8174–8179
Sowell ER, Trauner DA, Gamst A, Jernigan TL (2002) Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol 44:4–16
Toga AW, Thompson PM, Sowell E (2006) Mapping brain maturation. Trends Neurosci 29:148–159
Rothman DL (1994) 1H NMR studies of human brain metabolism and physiology. In: Gillies RJ (ed) NMR in physiology and biomedicine. Academic Press, San Diego, pp 353–372
Modica-Napolitano JS, Renshaw PF (2004) Ethanolamine and phospho- rethanolamine inhibit mitochondrial function in vitro: implications for mitochondrial dysfunction hypothesis in depression and bipolar disorder. Biol Psychiatry 55:273–277
Ongür D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF (2009) Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Res 172:44–48
Gangadhar BN, Jayakumar PN, Subbakrishna DK, Janakiramaiah N, Keshavan MS (2004) Basal ganglia high-energy phosphate metabolism in neuroleptic-naive patients with schizophrenia: a 31-phosphorus magnetic resonance spectroscopic study. Am J Psychiatry 161:1304–1306
Sung YH, Yurgelun-Todd DA, Shi XF, Kondo DG, Lundberg KJ, McGlade EC, Hellem TL, Huber RS, Fiedler KK, Harrell RE, Nickerson BR, Kim SE, Jeong EK, Renshaw PF (2013) Decreased frontal lobe phosphocreatine levels in meth- amphetamine users. Drug Alcohol Depend 129:102–109
Chang L, Ernst T, Speck O, Grob CS (2005) Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry 162:361–369
Sung YH, Cho SC, Hwang J, Kim SJ, Kim H, Bae S, Kim N, Chang KH, Daniels M, Renshaw PF, Lyoo IK (2007) Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study. Drug Alcohol Depend 88:28–35
Taylor MJ, Schweinsburg BC, Alhassoon OM, Gongvatana A, Brown GG, Young-Casey C, Letendre SL, Grant I, HNRC Group (2007) Effects of human immunodeficiency virus and methamphetamine on cerebral metabolites measured with magnetic resonance spectroscopy. J Neurovirol 13:150–159
Sailasuta N, Abulseoud O, Hernandez M, Haghani P, Ross BD (2010) Metabolic abnormalities in abstinent methamphetamine dependent subjects. Subst Abuse 2010:9–20
Chang L, Ernst T, Leonido-Yee M, Walot I, Singer E (1999) Cerebral metabolite abnormalities correlate with clinical severity of HIV-cognitive motor complex. Neurology 52:100–108
Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D (2005) Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain 128:1016–1025
Ross B, Bluml S (2001) Magnetic resonance spectroscopy of the human brain. Anat Rec 265:54–84
Videen JS, Michaelis T, Pinto P, Ross BD (1995) Human cerebral osmolytes during chronic hyponatremia. A proton magnetic resonance spectroscopy study. J Clin Invest 95:788–793
Chang L, Friedman J, Ernst T, Zhong K, Tsopelas ND, Davis K (2007) Brain metabolite abnormalities in the white matter of elderly schizophrenic subjects: implication for glial dysfunction. Biol Psychiatry 62:1396–1404
Tayoshi S, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, Iga J, Nakataki M, Ueno S, Harada M, Ohmori T (2009) Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS). Schizophr Res 108:69–77
Shimon H, Sobolev Y, Davidson M, Haroutunian V, Belmaker RH, Agam G (1998) Inositol levels are decreased in postmortem brain of schizophrenic patients. Biol Psychiatry 44:428–432
Rudkin TM, Arnold DL (1999) Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch Neurol 56:919–926
Moore GJ, Galloway MP (2002) Magnetic resonance spectroscopy: neurochemistry and treatment effects in affective disorders. Psychopharmacol Bull 36:5–23
Kirov II, Patil V, Babb JS, Rusinek H, Herbert J, Gonen O (2009) MR spectroscopy indicates diffuse multiple sclerosis activity during remission. J Neurol Neurosurg Psychiatr 80:1330–1336
Ohrmann P, Siegmund A, Suslow T, Pedersen A, Spitzberg K, Kersting A, Rothermundt M, Arolt V, Heindel W, Pfleiderer B (2007) Cognitive impairment and in vivo metabolites in first-episode neuroleptic-naive and chronic medicated schizophrenic patients: a proton magnetic resonance spectroscopy study. J Psychiatr Res 41:625–634
Yoo SY, Yeon S, Choi CH, Kang DH, Lee JM, Shin NY, Jung WH, Choi JS, Jang DP, Kwon JS (2009) Proton magnetic resonance spectroscopy in subjects with high genetic risk of schizophrenia: investigation of anterior cingulate, dorsolateral prefrontal cortex and thalamus. Schizophr Res 111:86–93
Keshavan MS, Dick RM, Diwadkar VA, Montrose DM, Prasad KM, Stanley JA (2009) Striatal metabolic alterations in non-psychotic adolescent offspring at risk for schizophrenia: a 1H spectroscopy study. Schizophr Res 115:88–93
Acknowledgments
This study was supported in part by a Grant from the National Natural Science Foundation of China (30930027) and a postdoctoral research fellowship (G.Y.) from Li Ka Shing foundation.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yan, G., Xuan, Y., Dai, Z. et al. Brain Metabolite Changes in Subcortical Regions After Exposure to Cuprizone for 6 Weeks: Potential Implications for Schizophrenia. Neurochem Res 40, 49–58 (2015). https://doi.org/10.1007/s11064-014-1464-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1464-2