Abstract
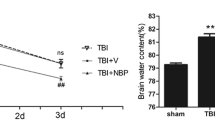
Hydrogen sulfide (H2S) is a lipid-soluble, endogenously produced gaseous messenger molecule collectively known as gasotransmitter. Over the last several decades, gasotransmitters have emerged as potent cytoprotective mediators in various models of tissue and cellular injury. In this study, we performed a weight-drop traumatic brain injury (TBI) model in adult mice and investigated changes of H2S and its possible role in the pathogenesis after TBI. Expression of Cystathionine-β-synthase (CBS) mRNA as H2S-producing enzymes in mouse brain was determined by reverse transcriptase-polymerase chain reaction (RT-PCR). From the results of RT-PCR, it was found that the expression of CBS was down-regulated in mouse brain cortex and hippocampus after brain injury. Western blot analysis revealed that CBS was present in normal mouse brain cortex and the hippocampus. It gradually decreased, reached its lowest level and then increased. Hydrogen sulfide in the cortex and hippocampus exhibited dynamic changes after brain injury, in parallel with CBS mRNA and protein expression. Moreover, pretreatment with the H2S donor (NaHS) could protect the neuron against the injury induced by TBI. Noticeably, the H2S donor NaHS could reduce TBI-induced injury assessed with lesion volume. These data suggested that H2S may have a therapeutic potential against neuron damage.






Similar content being viewed by others
References
Werner C, Engelhard K (2007) Pathophysiology of traumatic brain injury. Br J Anaesth 99:4–9
Greve MW, Zink BJ (2009) Pathophysiology of traumatic brain injury. Mount Sinai J Med New York 76:97–104
Zhao J, Pati S, Redell JB et al (2012) Caffeic acid phenethyl ester protects blood–brain barrier integrity and reduces contusion volume in rodent models of traumatic brain injury. J Neurotrauma 29:1209–1218
Sande A, West C (2010) Traumatic brain injury: a review of pathophysiology and management. J Vet Emerg Crit Care 20:177–190
McAllister TW (2008) Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry 7:3–10
McAllister TW (2011) Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci 13:287–300
Dash PK, Johnson D, Clark J et al (2011) Involvement of the glycogen synthase kinase-3 signaling pathway in TBI pathology and neurocognitive outcome. PLoS ONE 6:e24648
Feeser VR, Loria RM (2011) Modulation of traumatic brain injury using progesterone and the role of glial cells on its neuroprotective actions. J Neuroimmunol 237:4–12
Huang T, Solano J, He D et al (2009) Traumatic injury activates MAP kinases in astrocytes: mechanisms of hypothermia and hyperthermia. J Neurotrauma 26:1535–1545
Stoica BA, Faden AI (2010) Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics 7:3–12
Kimura H (2010) Hydrogen sulfide: from brain to gut. Antioxid Redox Signal 12:1111–1123
Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16:1066–1071
Goodwin LR, Francom D, Dieken FP et al (1989) Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol 13:105–109
Robert K, Vialard F, Thiery E et al (2003) Expression of the cystathionine beta synthase (CBS) gene during mouse development and immunolocalization in adult brain. J Histochem Cytochem 51:363–371
Enokido Y, Suzuki E, Iwasawa K et al (2005) Cystathionine beta-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J 19:1854–1856
Awata S, Nakayama K, Sato A et al (1993) Changes in cystathionine gamma-lyase levels in rat liver during lactation. Biochem Mol Biol Int 31:185–191
Qu K, Chen CP, Halliwell B et al (2006) Hydrogen sulfide is a mediator of cerebral ischemic damage. Stroke 37:889–893
Awata S, Nakayama K, Suzuki I et al (1995) Changes in cystathionine gamma-lyase in various regions of rat brain during development. Biochem Mol Biol Int 35:1331–1338
Nagai Y, Tsugane M, Oka J et al (2004) Hydrogen sulfide induces calcium waves in astrocytes. FASEB J 18:557–559
Geng B, Chang L, Pan C et al (2004) Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun 318:756–763
Han Y, Qin J, Chang X et al (2005) Modulating effect of hydrogen sulfide on gamma-aminobutyric acid B receptor in recurrent febrile seizures in rats. Neurosci Res 53:216–219
Lee SW, Hu YS, Hu LF et al (2006) Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia 54:116–124
Tay AS, Hu LF, Lu M et al (2010) Hydrogen sulfide protects neurons against hypoxic injury via stimulation of ATP-sensitive potassium channel/protein kinase C/extracellular signal-regulated kinase/heat shock protein 90 pathway. Neuroscience 167:277–286
Hu LF, Lu M, Wu ZY et al (2009) Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol Pharmacol 75:27–34
Liu YY, Sparatore A, Del Soldato P et al (2011) ACS84, a novel hydrogen sulfide-releasing compound, protects against amyloid beta-induced cell cytotoxicity. Neurochem Int 58:591–598
Liu YY, Bian JS (2010) Hydrogen sulfide protects amyloid-beta induced cell toxicity in microglia. J Alzheimer’s Dis 22:1189–1200
Schreier SM, Muellner MK, Steinkellner H et al (2010) Hydrogen sulfide scavenges the cytotoxic lipid oxidation product 4-HNE. Neurotox Res 17:249–256
Tang XQ, Shen XT, Huang YE et al (2010) Hydrogen sulfide antagonizes homocysteine-induced neurotoxicity in PC12 cells. Neurosci Res 68:241–249
Yin WL, He JQ, Hu B et al (2009) Hydrogen sulfide inhibits MPP(+)-induced apoptosis in PC12 cells. Life Sci 85:269–275
Xie L, Tiong CX, Bian JS (2012) Hydrogen sulfide protects SH-SY5Y cells against 6-hydroxydopamine-induced endoplasmic reticulum stress. Am J Physiol Cell Physiol 303:C81–C91
Shao JL, Wan XH, Chen Y et al (2011) H2S protects hippocampal neurons from anoxia-reoxygenation through cAMP-mediated PI3 K/Akt/p70S6 K cell-survival signaling pathways. J Mol Neurosci 43:453–460
Meng JL, Mei WY, Dong YF et al (2011) Heat shock protein 90 mediates cytoprotection by H(2)S against chemical hypoxia-induced injury in PC12 cells. Clin Exp Pharmacol Physiol 38:42–49
Qin ZH, Chen RW, Wang Y et al (1999) Nuclear factor kappaB nuclear translocation upregulates c-Myc and p53 expression during NMDA receptor-mediated apoptosis in rat striatum. J Neurosci 19:4023–4033
Feeney DM, Boyeson MG, Linn RT et al (1981) Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res 211:67–77
Luo CL, Chen XP, Yang R et al (2010) Cathepsin B contributes to traumatic brain injury-induced cell death through a mitochondria-mediated apoptotic pathway. J Neurosci Res 88:2847–2858
Kimura H (2000) Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun 267:129–133
Luo CL, Li BX, Li QQ et al (2011) Autophagy is involved in traumatic brain injury-induced cell death and contributes to functional outcome deficits in mice. Neuroscience 184:54–63
Zhang M, Shan H, Gu Z et al (2012) Increased expression of calcium/calmodulin-dependent protein kinase type II subunit delta after rat traumatic brain injury. J Mol Neurosci 46:631–643
Ishigami M, Hiraki K, Umemura K et al (2009) A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxid Redox Signal 11:205–214
Shibuya N, Tanaka M, Yoshida M et al (2009) 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11:703–714
McIntosh TK, Faden AI, Bendall MR et al (1987) Traumatic brain injury in the rat: alterations in brain lactate and pH as characterized by 1H and 31P nuclear magnetic resonance. J Neurochem 49:1530–1540
Plesnila N, Haberstok J, Peters J et al (1999) Effect of lactacidosis on cell volume and intracellular pH of astrocytes. J Neurotrauma 16:831–841
Shono Y, Kamouchi M, Kitazono T et al (2010) Change in intracellular pH causes the toxic Ca2+ entry via NCX1 in neuron- and glia-derived cells. Cell Mol Neurobiol 30:453–460
Kimura H (2011) Hydrogen sulfide: its production, release and functions. Amino Acids 41:113–121
Renga B (2011) Hydrogen sulfide generation in mammals: the molecular biology of cystathionine-beta-synthase (CBS) and cystathionine-gamma-lyase (CSE). Inflamm Allergy Drug Targets 10:85–91
Ignoul S, Eggermont J (2005) CBS domains: structure, function, and pathology in human proteins. Am J Physiol Cell Physiol 289:C1369–C1378
Tang B, Mustafa A, Gupta S et al (2010) Methionine-deficient diet induces post-transcriptional downregulation of cystathionine beta-synthase. Nutrition 26:1170–1175
Ratnam S, Maclean KN, Jacobs RL et al (2002) Hormonal regulation of cystathionine beta-synthase expression in liver. J Biol Chem 277:42912–42918
Goldstein JL, Campbell BK, Gartler SM (1972) Cystathionine synthase activity in human lymphocytes: induction by phytohemagglutinin. J Clin Invest 51:1034–1037
Maclean KN, Gaustadnes M, Oliveriusova J et al (2002) High homocysteine and thrombosis without connective tissue disorders are associated with a novel class of cystathionine beta-synthase (CBS) mutations. Hum Mutat 19:641–655
Lee M, Schwab C, Yu S et al (2009) Astrocytes produce the antiinflammatory and neuroprotective agent hydrogen sulfide. Neurobiol Aging 30:1523–1534
Ray SK, Dixon CE, Banik NL (2002) Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol Histopathol 17:1137–1152
Lagraoui M, Latoche JR, Cartwright NG et al (2012) Controlled cortical impact and craniotomy induce strikingly similar profiles of inflammatory gene expression, but with distinct kinetics. Front Neurol 3:155
Scheff SW, Ansari MA, Roberts KN (2012) Neuroprotective effect of Pycnogenol(R) following traumatic brain injury. Exp Neurol 239C:183–191
Banerjee R, Zou CG (2005) Redox regulation and reaction mechanism of human cystathionine-beta-synthase: a PLP-dependent hemesensor protein. Arch Biochem Biophys 433:144–156
Vitvitsky V, Thomas M, Ghorpade A et al (2006) A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem 281:35785–35793
Zhong WX, Wang YB, Peng L et al (2012) Lanthionine synthetase C-like PROTEIN 1 Interacts with and inhibits cystathionine beta-synthase: a target for neuronal antioxidant defense. J Biol Chem 287:34189–34201
Rhoads AR, Friedberg F (1997) Sequence motifs for calmodulin recognition. FASEB J 11:331–340
Eto K et al (2002) Hydrogen sulfide is produced in response to neuronal excitation. J Neurosci 22:3386–3391
Folkerts MM, Parks EA, Dedman JR et al (2007) Phosphorylation of calcium calmodulin-dependent protein kinase II following lateral fluid percussion brain injury in rats. J Neurotrauma 24:638–650
Osipov RM, Robich MP, Feng J et al (2009) Effect of hydrogen sulfide in a porcine model of myocardial ischemia-reperfusion: comparison of different administration regimens and characterization of the cellular mechanisms of protection. J Cardiovasc Pharmacol 54:287–297
Sodha NR, Clements RT, Feng J et al (2008) The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg 33:906–913
Quintana M, Kahan T, Hjemdahl P (2004) Pharmacological prevention of reperfusion injury in acute myocardial infarction. A potential role for adenosine as a therapeutic agent. Am J Cardiovasc Drugs 4:159–167
Bian JS, Yong QC, Pan TT et al (2006) Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther 316:670–678
Kimura Y, Kimura H (2004) Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18:1165–1167
Elrod JW, Calvert JW, Morrison J et al (2007) Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104:15560–15565
Kimura Y, Dargusch R, Schubert D et al (2006) Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal 8:661–670
Tyagi N, Moshal KS, Sen U et al (2009) H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid Redox Signal 11:25–33
Ji Y, Pang QF, Xu G et al (2008) Exogenous hydrogen sulfide postconditioning protects isolated rat hearts against ischemia-reperfusion injury. Eur J Pharmacol 587:1–7
Ren C, Du A, Li D et al (2010) Dynamic change of hydrogen sulfide during global cerebral ischemia-reperfusion and its effect in rats. Brain Res 1345:197–205
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30872666, No. 81172911 and No. 81271379); Natural Science Foundation of Medical College of Nantong University (No.Y201003); The Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); The Colleges and Universities in Jiangsu Province Plans to Graduate Research and Innovation (CXLX12_0824).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Mingyang Zhang and Haiyan Shan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, M., Shan, H., Wang, T. et al. Dynamic Change of Hydrogen Sulfide After Traumatic Brain Injury and its Effect in Mice. Neurochem Res 38, 714–725 (2013). https://doi.org/10.1007/s11064-013-0969-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-0969-4