Abstract
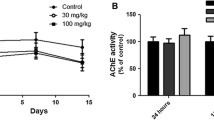
Sarin is a toxic organophosphorus (OP) nerve agent that has been reported to cause long-term alterations in behavioral and neuropsychological processes. The present study was designed to investigate the effect of low dose sarin exposure on the monoamine neurotransmitter systems in various brain regions of mice. The rationale was to expand our knowledge about the noncholinergic neurochemical alterations associated with low dose exposure to this cholinesterase inhibitor. We analyzed the levels of monoamines and their metabolites in different brain areas after exposure of male C57BL/6 mice to a subclinical dose of sarin (0.4 LD50). Mice did not show any signs of cholinergic toxicity or pathological changes in brain tissue. At 1, 4 and 8 weeks post-sarin exposure brains were collected for neurochemical analysis. A significant decrease in the dopamine (DA) turnover, as measured by the metabolite to parent ratio, was observed in the frontal cerebral cortex (FC) at all time points tested. DA turnover was significantly increased in the amygdala at 4 weeks but not at 1 or 8 weeks after exposure. The caudate nucleus displayed a decrease in DA turnover at 1 week but no significant change was observed at 4 and 8 weeks suggesting a reversible effect. In addition to this, serotonin (5-HT) levels were transiently altered at various time points in all the brain regions studied (increase in FC, caudate nucleus and decrease in amygdala). Since there were no signs of cholinergic toxicity or cell death after sarin exposure, different non-cholinergic mechanisms may be involved in regulating these effects. Our results demonstrate that non-symptomatic dose of OP nerve agent sarin has potent long-term, region-specific effects on the monoaminergic neurotransmitter systems. Data also suggests differential effects of sarin on the various DA projections. These neurochemical alterations could be associated with long term behavioral and neuropsychological changes associated with low dose OP exposure.





Similar content being viewed by others
References
Marrs TC, Maynard RL, Sidell FR (1996) Chemical warfare agents. Toxicology and treatment. Wiley, New York
Taylor P (2001) Anticholinesterase agents. In: Limbird LE, Hardman JG, Gilman AG (eds) Goodman and Gilman’s the pharmacological basis of therapeutics. McGraw Hill, New-York, pp 175–191
McDonough JH, Shih TM (1997) Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev 21(5):559–579
Shih TM, Duniho SM, McDonough JH (2003) Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol 188(2):69–80
Pope C, Karanth S, Liu J (2005) Pharmacology and toxicology of cholinesterase inhibitors: uses and misuses of a common mechanism of action. Elsevier, Amsterdam
Haley RW (1999) Is there a Gulf War syndrome? Lancet 354(9190):1645–1646
Nakajima T et al (1999) Sequelae of sarin toxicity at one and three years after exposure in Matsumoto, Japan. J Epidemiol 9(5):337–343
Barr DB et al (2004) Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environ Health Perspect 112(2):186–200
Chao LL et al (2010) Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicology 31(5):493–501
IOM (1995) Health consequences of service during the Persian Gulf War: initial findings and recommendations for immediate action. National Academy Press, Washington, DC
Couzin J (2004) Epidemiology. VA advisers link Gulf War illnesses to neurotoxins. Science 306(5693):26–27
Lashof JC, Arthur Caplan JB, Cross TP, Custis D, Hamburg DA, Johnson JA, Knox M, Landrigan PJ, Larson EL, Rios R, Taylor AK (1996) Presidential advisory committee on Gulf War Veterans’ illnesses, in final report. Washington, DC
Stephens R et al (1995) Neuropsychological effects of long-term exposure to organophosphates in sheep dip. Lancet 345(8958):1135–1139
Rosenstock L et al (1991) Chronic central-nervous system effects of acuteorganophosphate pesticide intoxication. Lancet 338(8761):223–227
Bazylewicz-Walczak B, Majczakowa W, Szymczak M (1999) Behavioral effects of occupational exposure to organophosphorous pesticides in female greenhouse planting workers. Neurotoxicology 20(5):819–826
Farahat TM et al (2003) Neurobehavioural effects among workers occupationally exposed to organophosphorous pesticides. Occup Environ Med 60(4):279–286
Bouchard MF et al (2010) Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 125(6):e1270–e1277
Bouchard MF et al (2011) Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect 119(8):1189–1195
Engel SM et al (2011) Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect 119(8):1182–1188
Scremin OU et al (2003) Delayed neurologic and behavioral effects of subtoxic doses of cholinesterase inhibitors. J Pharmacol Exp Ther 304(3):1111–1119
Russell RW et al (1986) Behavioral, neurochemical and physiological effects of repeated exposures to subsymptomatic levels of the anticholinesterase, soman. Neurobehav Toxicol Teratol 8(6):675–685
Mach M et al (2008) Delayed behavioral and endocrine effects of sarin and stress exposure in mice. J Appl Toxicol 28(2):132–139
Mamczarz J et al (2010) An acute exposure to a sub-lethal dose of soman triggers anxiety-related behavior in guinea pigs: interactions with acute restraint. Neurotoxicology 31(1):77–84
Morris M, Key MP, Farah V (2007) Sarin produces delayed cardiac and central autonomic changes. Exp Neurol 203(1):110–115
Kassa J, Koupilova M, Vachek J (2001) The influence of low-level sarin inhalation exposure on spatial memory in rats. Pharmacol Biochem Behav 70(1):175–179
Shih TM, Hulet SW, McDonough JH (2006) The effects of repeated low-dose sarin exposure. Toxicol Appl Pharmacol 215(2):119–134
Burchfiel JL, Duffy FH (1982) Organophosphate neurotoxicity: chronic effects of sarin on the electroencephalogram of monkey and man. Neurobehav Toxicol Teratol 4(6):767–778
Aldridge JE et al (2005) Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect 113(8):1027–1031
Kant GJ, Kenion CC, Meyerhoff JL (1984) Effects of diisopropylfluorophosphate (DFP) and other cholinergic agents on release of endogenous dopamine from rat brain striatum in vitro. Biochem Pharmacol 33(11):1823–1825
Coudraylucas C et al (1987) Changes in brain monoamine content and metabolism induced by paraoxon and soman intoxication—effect of atropine. Xenobiotica 17(9):1131–1138
Christin D et al (2008) Effects of repeated low-dose soman exposure on monoamine levels in different brain structures in mice. Neurochem Res 33(5):919–926
Eells JB, Brown T (2009) Repeated developmental exposure to chlorpyrifos and methyl parathion causes persistent alterations in nicotinic acetylcholine subunit mRNA expression with chlorpyrifos altering dopamine metabolite levels. Neurotoxicol Teratol 31(2):98–103
Moreno M et al (2008) Long-term monoamine changes in the striatum and nucleus accumbens after acute chlorpyrifos exposure. Toxicol Lett 176(2):162–167
Casida JE, Quistad GB (2004) Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol 17(8):983–998
Rocha ES et al (1999) Low concentrations of the organophosphate VX affect spontaneous and evoked transmitter release from hippocampal neurons: toxicological relevance of cholinesterase-independent actions. Toxicol Appl Pharmacol 159(1):31–40
Duysen EG et al (2001) Evidence for nonacetylcholinesterase targets of organophosphorus nerve agent: supersensitivity of acetylcholinesterase knockout mouse to VX lethality. J Pharmacol Exp Ther 299(2):528–535
Cooper JR, Bloom FE, Roth RH (1982) Catecholamines II—CNS aspects. In: The biochemical basis of neuropharmacology. Oxford University Press, New York, pp 173–222
Feldman RS, Meyer JS, Quenzer LF (1997) Catecholamines. In: Farley P (ed) Neuropsychopharmacology. Sinauer Associates Inc., Sunderland, pp 277–344
Haley RW (1997) Is there a Gulf War syndrome? Searching for syndromes by factor analysis of symptoms (vol 277, pg 215, 1997). JAMA 278(5):388
Maxwell DM, Brecht KM, O’Neill BL (1987) The effect of carboxylesterase inhibition on interspecies differences in soman toxicity. Toxicol Lett 39(1):35–42
Garrett TL et al (2010) A murine model for sarin exposure using the carboxylesterase inhibitor CBDP. Neurotoxicology 31(5):502–508
Ellman GL et al (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Schmued LC et al (2005) Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res 1035(1):24–31
Schmued LC, Albertson C, Slikker W (1997) Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res 751(1):37–46
Keith BJ, Franklin GP (2008) The mouse brain in stereotaxic coordinates, 3rd edn. Academic Press, New York
Harvey AT et al (1994) Monoamine activity in anterior hypothalamus of guinea pig pups separated from their mothers. Behav Neurosci 108(1):171–176
Lucot JB et al (2005) Measurement of plasma catecholamines in small samples from mice. J Pharmacol Toxicol Methods 52(2):274–277
Bloch-Shilderman E et al (2008) Subchronic exposure to low-doses of the nerve agent VX: physiological, behavioral, histopathological and neurochemical studies. Toxicol Appl Pharmacol 231(1):17–23
Choudhary S et al (2002) Possible involvement of dopaminergic neurotransmitter system in dichlorvos induced delayed neurotoxicity. J Biochem Mol Biol Biophys 6(1):29–36
Fuxe K et al (1974) Origin of dopamine nerve-terminals in limbic and frontal cortex—evidence for meso-cortico dopamine neurons. Brain Res 82(2):349–355
Oades RD, Halliday GM (1987) Ventral tegmental (A10) system—neurobiology. 1. Anatomy and connectivity. Brain Res Rev 12(2):117–165
White FJ (1996) Synaptic regulation of mesocorticolimbic dopamine neurons. Annu Rev Neurosci 19:405–436
Fallon JH, Koziell DA, Moore RY (1978) Catecholamine innervation of basal forebrain II. Amygdala, suprarhinal cortex and entorhinal cortex. J Compar Neurol 180(3):509–531
Inglis FM, Moghaddam B (1999) Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem 72(3):1088–1094
Hasue RH, Shammah-Lagnado SJ (2002) Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Compar Neurol 454(1):15–33
Padilla S et al (2005) Neurochemical effects of chronic dietary and repeated high-level acute exposure to chlorpyrifos in rats. Toxicol Sci 88(1):161–171
Pope CN et al (1992) Long-term neurochemical and behavioral effects induced by acute chlorpyrifos treatment. Pharmacol Biochem Behav 42(2):251–256
Rausch JL et al (1985) Physostigmine effects on serotonin uptake in human-blood platelets. Eur J Pharmacol 109(1):91–96
Oquendo MA, Mann JJ (2000) The biology of impulsivity and suicidality. Psychiatr Clin North Am 23(1):11–25
Roldan-Tapia L et al (2006) Neuropsychological sequelae from acute poisoning and long-term exposure to carbamate and organophosphate pesticides. Neurotoxicol Teratol 28(6):694–703
London L et al (2005) Suicide and exposure to organophosphate insecticides: cause or effect? Am J Ind Med 47(4):308–321
Slotkin TA, Seidler FJ (2007) Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull 72(4–6):232–274
Acknowledgments
This work was supported by a grant from the Gulf War Veterans Illnesses Program (GW060050). We thank all the members of the author’s laboratory particularly Kate Irwin, Kaushal Joshi, Ajay Sharma, Abigail Schwartz and Christine Rapp for their contribution to the project.
Conflict of interest
The authors declare no conflict of interest associated with this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oswal, D.P., Garrett, T.L., Morris, M. et al. Low-Dose Sarin Exposure Produces Long Term Changes in Brain Neurochemistry of Mice. Neurochem Res 38, 108–116 (2013). https://doi.org/10.1007/s11064-012-0896-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0896-9