Abstract
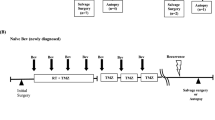
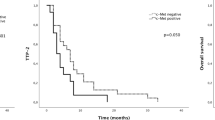
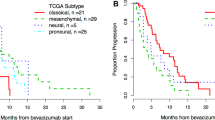
Angiogenesis is one of the key features of glioblastoma (GB). However, the use of anti-angiogenic therapies directed against vascular endothelial growth factor (VEGF) is limited by primary or acquired resistance. MET/HGF and PlGF signaling are involved in potential alternative escape mechanisms to VEGF pathway. Our objective was to explore the potential changes of MET/HGF and PlGF expression, comparing initial diagnosis and recurrence after radiotherapy-temozolomide (RT/TMZ). Paired frozen tumors from both initial and recurrent surgery after radio-chemotherapy were available for 28 patients. RNA expressions of PlGF, MET, and HGF genes were analyzed by RT-qPCR. PlGF expression significantly decreased at recurrence (p = 0.021), and expression of MET showed a significant increase (p = 0.011) at recurrence. RNA expressions of MET and HGF significantly correlated both at baseline and recurrence (baseline: p = 0.005; recurrence: p = 0.019). Evolutive profile (increasing versus decreasing expression at recurrence) of MET was associated with PFS (p = 0.002) and OS (p = 0.022) at recurrence, while the evolutive profile of HGF was associated with PFS at relapse (p = 0.049). Recurrence of GB after chemo-radiation could be associated with a variation in PlGF and MET expression. These results contribute to suggest a modification of the GB angiogenic process between initial diagnosis and recurrence.



Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Plate KH, Breier G, Weich HA, Risau W (1992) Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature 359:845–848. doi:10.1038/359845a0
Carmeliet P, Moons L, Luttun A et al (2001) Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 7:575–583. doi:10.1038/87904
Fischer C, Mazzone M, Jonckx B, Carmeliet P (2008) FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer 8:942–956. doi:10.1038/nrc2524
Schneider K, Weyerbrock A, Doostkam S et al (2015) Lack of evidence for PlGF mediating the tumor resistance after anti-angiogenic therapy in malignant gliomas. J Neurooncol 121:269–278. doi:10.1007/s11060-014-1647-3
Jahangiri A, De Lay M, Miller LM et al (2013) Gene expression profile identifies tyrosine kinase c-Met as a targetable mediator of antiangiogenic therapy resistance. Clin Cancer Res 19:1773–1783. doi:10.1158/1078-0432.CCR-12-1281
Koochekpour S, Jeffers M, Rulong S et al (1997) Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res 57:5391–5398
Xie C-R, Sun H, Wang F-Q et al (2015) Integrated analysis of gene expression and DNA methylation changes induced by hepatocyte growth factor in human hepatocytes. Mol Med Rep 12:4250–4258. doi:10.3892/mmr.2015.3974
Siddique AN, Nunna S, Rajavelu A et al (2013) Targeted methylation and gene silencing of VEGF-A in human cells by using a designed Dnmt3a-Dnmt3L single-chain fusion protein with increased DNA methylation activity. J Mol Biol 425:479–491. doi:10.1016/j.jmb.2012.11.038
Rajabi H, Tagde A, Alam M et al (2016) DNA methylation by DNMT1 and DNMT3b methyltransferases is driven by the MUC1-C oncoprotein in human carcinoma cells. Oncogene. doi:10.1038/onc.2016.180
Tagde A, Rajabi H, Stroopinsky D et al (2016) MUC1-C induces DNA methyltransferase 1 and represses tumor suppressor genes in acute myeloid leukemia. Oncotarget. doi:10.18632/oncotarget.9777
Baudino TA, McKay C, Pendeville-Samain H et al (2002) c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev 16:2530–2543. doi:10.1101/gad.1024602
Mezquita P, Parghi SS, Brandvold KA, Ruddell A (2005) Myc regulates VEGF production in B cells by stimulating initiation of VEGF mRNA translation. Oncogene 24:889–901. doi:10.1038/sj.onc.1208251
Bouillez A, Rajabi H, Pitroda S et al (2016) Inhibition of MUC1-C suppresses MYC expression and attenuates malignant growth in KRAS mutant lung adenocarcinomas. Cancer Res 76:1538–1548. doi:10.1158/0008-5472.CAN-15-1804
Tagde A, Rajabi H, Bouillez A et al (2016) MUC1-C drives MYC in multiple myeloma. Blood 127:2587–2597. doi:10.1182/blood-2015-07-659151
Taal W, Oosterkamp HM, Walenkamp AME et al (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15:943–953. doi:10.1016/S1470-2045(14)70314-6
Chinot OL, Wick W, Mason W et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 370:709–722. doi:10.1056/NEJMoa1308345
de Groot JF, Lamborn KR, Chang SM et al (2011) Phase II study of aflibercept in recurrent malignant glioma: a North American brain tumor consortium study. J Clin Oncol 29:2689–2695. doi:10.1200/JCO.2010.34.1636
Wen PY, Schiff D, Cloughesy TF et al (2011) A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro-Oncology 13:437–446. doi:10.1093/neuonc/noq198
Lassen U, Chinot OL, McBain C et al (2015) Phase 1 dose-escalation study of the antiplacental growth factor monoclonal antibody RO5323441 combined with bevacizumab in patients with recurrent glioblastoma. Neuro-Oncology 17:1007–1015. doi:10.1093/neuonc/nov019
Tabouret E, Cauvin C, Fuentes S et al (2013) Reassessment of scoring systems and prognostic factors for metastatic spinal cord compression. Spine J. doi:10.1016/j.spinee.2013.06.036
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Figarella-Branger D, Mokhtari K, Dehais C et al (2014) Mitotic index, microvascular proliferation, and necrosis define 3 groups of 1p/19q codeleted anaplastic oligodendrogliomas associated with different genomic alterations. Neuro-Oncology 16:1244–1254. doi:10.1093/neuonc/nou047
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
Schroeder A, Mueller O, Stocker S et al (2006) The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol 7:3. doi:10.1186/1471-2199-7-3
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Valente V, Teixeira SA, Neder L et al (2009) Selection of suitable housekeeping genes for expression analysis in glioblastoma using quantitative RT-PCR. BMC Mol Biol 10:17. doi:10.1186/1471-2199-10-17
Hasegawa M, Takahashi H, Rajabi H et al (2016) Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget 7:11756–11769. doi:10.18632/oncotarget.7598
Takahashi H, Jin C, Rajabi H et al (2015) MUC1-C activates the TAK1 inflammatory pathway in colon cancer. Oncogene 34:5187–5197. doi:10.1038/onc.2014.442
Sonawane P, Cho HE, Tagde A et al (2014) Metabolic characteristics of 13-cis-retinoic acid (isotretinoin) and anti-tumour activity of the 13-cis-retinoic acid metabolite 4-oxo-13-cis-retinoic acid in neuroblastoma. Br J Pharmacol 171:5330–5344. doi:10.1111/bph.12846
Eder JP, Vande Woude GF, Boerner SA, LoRusso PM (2009) Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res 15:2207–2214. doi:10.1158/1078-0432.CCR-08-1306
Kim KJ, Li B, Winer J et al (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362:841–844. doi:10.1038/362841a0
Tabouret E, Tchoghandjian A, Denicolai E et al (2015) Recurrence of glioblastoma after radio-chemotherapy is associated with an angiogenic switch to the CXCL12-CXCR4 pathway. Oncotarget 6:11664–11675. doi:10.18632/oncotarget.3256
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473:298–307. doi:10.1038/nature10144
Awad AJ, Burns TC, Zhang Y, Abounader R (2014) Targeting MET for glioma therapy. Neurosurg Focus 37:E10. doi:10.3171/2014.9.FOCUS14520
Kong D-S, Song S-Y, Kim D-H et al (2009) Prognostic significance of c-Met expression in glioblastomas. Cancer 115:140–148. doi:10.1002/cncr.23972
Acknowledgments
SIRIC Grant: INCa-DGOS-Inserm 6038, ARTC-Sud, APHM Tumor Bank (authorization number AC-2013–1786).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tabouret, E., Denicolai, E., Delfino, C. et al. Changes in PlGF and MET-HGF expressions in paired initial and recurrent glioblastoma. J Neurooncol 130, 431–437 (2016). https://doi.org/10.1007/s11060-016-2251-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-016-2251-5