Abstract
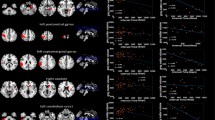
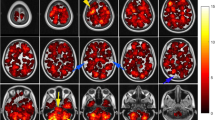
The aim of this study was to test by means of a voxel-based approach the hypothesis that there is a different spatial distribution of brain metastases (BM) and white matter hyperintensities (WMH) and that the presence of WMH affects the location of BM in lung and non-lung cancer patients. Two-hundred consecutive cancer patients at first diagnosis of BM were included. Images were acquired using a 1.5 Tesla MRI system (Magnetom Avanto B13, Siemens, Erlangen, Germany). Axial FLAIR T2 weighted images and gadolinium-enhanced T1 weighted images were post-processed for segmentation, co-registration and analysis. Binary lesion masks were created for WMH and BM, using Volumes of Interest. Lesion probability maps were generated and the voxel-based lesion-symptom mapping approach was used to model each voxel and to calculate a non parametric statistics (Brunner–Munzel test) describing the differences between the groups. In the lung cancer group we found higher frequency of BM in WMH− than in WMH+ patients in the occipital lobe and the cerebellum. In contrast, BM were more frequent in the right frontal lobe in WMH+ than in WMH− patients. We suggest that there exists an inverse brain spatial distribution between WMH and BM. In lung cancer patients, the presence of WMH seems to shift the distribution of BM toward locations different than what it is expected based on primary tumor.



Similar content being viewed by others
References
Tabarés-Seisdedos R, Rubenstein JL (2013) Inverse cancer comorbidity: a serendipitous opportunity to gain insight into CNS disorders. Nat Rev Neurosci 14:293–304
Ibáñez K, Boullosa C, Tabarés-Seisdedos R, Baudot A, Valencia A (2014) Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genet 10(2):e1004173
Vernooij MW, Ikram MA, Tanghe HL et al (2007) Incidental findings on brain MRI in the general population. N Engl J Med 357(18):1821–1828
Schmidt R, Hayn M, Fazekas F, Kapeller P, Esterbauer H (1996) Magnetic resonance imaging white matter hyperintensities in clinically normal elderly individuals: correlations with plasma concentrations of naturally occurring antioxidants. Stroke 27(11):2043–2047
Longstreth WT Jr, Manolio TA, Arnold A et al (1996) Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the Cardiovascular Health Study. Stroke 27(8):1274–1282
Yue NC, Arnold AM, Longstreth WT Jr et al (1997) Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: data from the Cardiovascular Health Study. Radiology 202(1):33–39
Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ (2005) Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology 237(1):251–257
de Leeuw FE, de Groot JC, Bots ML et al (2000) Carotid atherosclerosis and cerebral white matter lesions in a population based magnetic resonance imaging study. J Neurol 247:291–296
Altaf N, Morgan PS, Moody A, MacSweeney ST, Gladman JR, Auer DP (2008) Brain white matter hyperintensities are associated with carotid intraplaque hemorrhage. Radiology 248(1):202–209
Yamauchi H, Fukuyama H, Nagahama Y et al (1999) Brain arteriolosclerosis and hemodynamic disturbance may induce leukoaraiosis. Neurology 53:1833–1838
Jefferson AL, Tate DF, Poppas A et al (2007) Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. J Am Geriatr Soc 55:1044–1048
Vogels RL, van der Flier WM, van Harten B et al (2007) Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail 9(10):1003–1009
Purandare N, Oude Voshaar RC, Burns A, Velupandian UM, McCollum C (2006) Paradoxical embolization: a potential cause of cerebral damage in Alzheimer’s disease? Neurol Res 28(6):679–684
Luzzi KJ, MacDonald IC, Schmidt EE et al (1998) Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 153(3):865–873
Hwang TL, Close TP, Grego JM, Brannon WL, Gonzales F (1996) Predilection of brain metastases in grey and white matter junction and vascular border zones. Cancer 77:1551–1555
Graf AH, Buchberger W, Langmayr H, Schmid KW (1988) Site preference of metastatic tumours of the brain. Virchows Arch A Pathol Anat Histopathol 412(5):493–498
Tsukada Y, Fouad A, Pickren JW, Lane WW (1983) Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer 52:2349–2354
Bender ET, Tomé WA (2011) Distribution of brain metastases: implications for non-uniform dose prescriptions. Br J Radiol 84(1003):649–658
Quattrocchi CC, Errante Y, Gaudino C et al (2012) Spatial brain distribution of intra-axial metastatic lesions in breast and lung cancer patients. J Neurooncol 110(1):79–87
Graesslin O, Abdulkarim BS, Coutant C et al (2010) Nomogram to predict subsequent brain metastasis in patients with metastatic breast cancer. J Clin Oncol 28(12):2032–2037
Albiges L, André F, Balleyguier C, Gomez-Abuin G, Chompret A, Delaloge S (2005) Spectrum of breast cancer metastasis in BRCA1 mutation carriers: highly increased incidence of brain metastases. Ann Oncol 16(11):1846–1847
Mujoomdar A, Austin JH, Malhotra R et al (2007) Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology 242(3):882–888
Mazzone PJ, Marchi N, Fazio V et al (2009) Small vessel ischemic disease of the brain and brain metastases in lung cancer patients. PLoS One 4(9):e7242
Quattrocchi CC, Errante Y, Mallio CA, Santini D, Tonini G, Zobel BB (2013) Brain metastatic volume and white matter lesions in advanced cancer patients. J Neurooncol 113(3):451–458
Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J (1995) A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage 2(2):89–101
Rostrup E, Gouw AA, Vrenken H et al (2012) The spatial distribution of age-related white matter changes as a function of vascular risk factors-results from the LADIS study. Neuroimage 60(3):1597–1607
Fazekas F, Schmidt R, Kleinert R, Kapeller P, Roob G, Flooh E (1998) The spectrum of age-associated brain abnormalities: their measurement and histopathological correlates. J Neural Transm Suppl 53:31–39
Wen W, Sachdev P (2004) The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. Neuroimage 22(1):144–154
Enzinger C, Smith S, Fazekas F et al (2006) Lesion probability maps of white matter hyperintensities in elderly individuals: results of the Austrian stroke prevention study. J Neurol 253(8):1064–1070
Hengel K, Sidhu G, Choi J et al (2013) Attributes of brain metastases from breast and lung cancer. Int J Clin Oncol 18(3):396–401
Hendrikse J, Van der Grond J, Lu H et al (2004) Flow territory mapping of the cerebral arteries with regional perfusion MRI. Stroke 35:882–887
Iadecola C (1993) Regulation of the cerebral microcirculation during neural activity: is nitric oxide the missing link? TINS 16:206–215
Gouw AA, Seewann A, Vrenken H et al (2008) Heterogeneity of white matter hyperintensities in Alzheimer’s disease: post-mortem quantitative MRI and neuropathology. Brain 131:3286–3298
Gouw AA, Seewann A, van der Flier WM et al (2011) Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry 82(2):126–135
Marstrand JR, Garde E, Rostrup E et al (2002) Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke 33(4):972–976
Lorger M, Felding-Habermann B (2010) Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol 176(6):2958–2971
Furutani K, Harada M, Mawlan M, Nishitani H (2008) Difference in enhancement between spin echo and 3-dimensional fast spoiled gradient recalled acquisition in steady state magnetic resonance imaging of brain metastasis at 3-T magnetic resonance imaging. J Comput Assist Tomogr 32(2):313–319
Ethical Standards
The study was conducted in accordance with the declaration of Helsinki and it followed the institutional ethical committee standards for observational studies.
Conflict of interest
The authors report no financial or other conflict of interest relevant to the subject of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Quattrocchi, C.C., Errante, Y., Mallio, C.A. et al. Inverse spatial distribution of brain metastases and white matter hyperintensities in advanced lung and non-lung cancer patients. J Neurooncol 120, 321–330 (2014). https://doi.org/10.1007/s11060-014-1554-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1554-7