Abstract
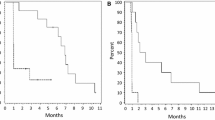
Treatment options are limited for recurrent glioblastoma (GBM). Verubulin is a microtubule destabilizer and vascular disrupting agent that achieve high brain concentration relative to plasma in animals. Adults with recurrent GBM who failed prior standard therapy were eligible. The primary endpoint was 1-month progression-free survival (PFS-1) for bevacizumab refractory (Group 2) and 6-month progression-free survival (PFS-6) for bevacizumab naïve patients (Group 1). Verubulin was administered at 3.3 mg/m2 as a 2-h intravenous infusion once weekly for 3 consecutive weeks in a 4-week cycle. The planned sample size was 34 subjects per cohort. 56 patients (37 men, 19 women) were enrolled, 31 in Group 1 and 25 in Group 2. The PFS-6 for Group 1 was 14 % and the PFS-1 for Group 2 was 20 %. Median survival from onset of treatment was 9.5 months in Group 1 and 3.4 months in Group 2. Best overall response was partial response (n = 3; 10 % in Group 1; n = 1; 4.2 % in Group 2) and stable disease (n = 7; 23 % in Group 1; n = 5; 21 % in Group 2). In Group 1, 38.7 % of patients experienced a serious adverse event; however only 3.2 % were potentially attributable to study drug. In Group 2, 44 % of patients experienced a serious adverse event although none were attributable to study drug. Accrual was terminated early for futility. Single agent verubulin, in this dose and schedule, is well tolerated, associated with moderate but tolerable toxicity but has limited activity in either bevacizumab naïve or refractory recurrent GBM.



Similar content being viewed by others
References
Ostrom QT, Gittleman H, Farah P, Onderacek A, Chen Y, Wolinsjy Y, Stroup NE, Kruchko C, Branholtz-Sloan J (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 15:ii1–ii56
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Eng J Med 352:987–996
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Grossman SA, Ye X, Piantadosi S, Desideri S, Nabors LB, Rosenfeld M, Fisher J (2010) Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res 16:2443–2449
Chamberlain M (2011) Evolving strategies: future treatment of glioblastoma. Expert Rev Neurother 11:519–532
Grimm S, Chamberlain MC (2012) State of art and perspectives on the treatment of glioblastoma. CNS Oncol 1:49–70
Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745
Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL et al (2008) Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70:779–787
Kargiotis O, Rao J, Kyritsis AP et al (2006) Mechanisms of angiogenesis in gliomas. J Neuro Oncol 78:281–293
Minchinton A, Tannock I (2006) Drug penetration in solid tumours. Nat Rev Cancer 6:583–592
Tozer GM, Kanthou C, Baguley BC et al (2005) Disrupting tumour blood vessels. Nat Rev Cancer 5(6):423–435
Siemann D, Bibby M, Dark GG, Dicker AP, Eskens FA, Horsman MR et al (2005) Differentiation and definition of vascular-targeted therapies. Clin Cancer Res 11(2 pt 1):416–420
Lippert J 3rd (2007) Vascular disrupting agents. Bioorg Med Chem 15:605–615
Kasibhatla S, Baichwal V, Cai SX, Roth B, Skvortsova I, Skvortsov S et al (2007) MPC-6827: A small-molecule inhibitor of microtubule formation that is not a substrate for multidrug resistance pumps. Can Res 67:5865–5871
Sirisoma N, Pervin A, Zhang H, Jiang S, Adam Willardsen J, Anderson MB et al (2010) Discovery of N-methyl-4-(4-methoxyanilino) quinazolines as potent apoptosis inducers. Structure-activity relationship of the quinazoline ring. Bioorg Med Chem Lett 20:2330–2334
Sirisoma N, Pervin A, Zhang H, Jiang S, Willardsen JA, Anderson MB et al (2009) Discovery of N-(4-methoxyphenyl)-N,2-dimethylquinazolin-4-amine, a potent apoptosis inducer and efficacious anticancer agent with high blood brain barrier penetration. J Med Chem 52:2341–2351
Pleiman C, Baichwal V, Bhoite L, et al (2007) Vascular disruption effects of MPC-6827. In: 98th Annual Meeting of the American Association for Cancer Research (AACR), 2007 in Los Angeles, California (Abstract/Poster)
Jessing K, Mauck K, Bradford C, et al (2005) MPC-6827, a small molecule inhibitor of microtubule formation with high brain penetration: absorption, distribution, metabolism, excretion, and clinical considerations. In: 96th Annual Meeting of the American Association for Cancer Research (AACR), 2005 in Anaheim, California (Abstract/Poster)
Yu MK (2009) MPC-6827: Anti-tumor activity in an orthotopic brain model and in combination with bevacizumab. In: The 2009 Joint Meeting of the Society for Neuro-Oncology (SNO) and the AANS/CNS Section, October 23, 2009 in New Orleans, LA
Jones JT (2009) MPC-6827: A potent tubulin binding and vascular disrupting agent with high brain penetration and anti-tumor activity in a mouse orthotopic glioma model. In: The 100th meeting of the American Association for Cancer Research (AACR), April 22, 2009 in Denver, CO
Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578
Lamborn KR, Yung WK, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM et al (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 10:162–170
Ballman KV, Buckner JC, Brown PD, Giannini C, Flynn PJ, LaPlant BR, Jaeckle KA (2007) The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro Oncol 9:29–38
Quant EC, Norden AD, Drappatz J, Muzikansky A, Doherty L, Lafrankie D et al (2007) Role of a second chemotherapy in recurrent malignant glioma patients who progress on bevacizumab. Neuro Oncol 11:550–555
Chamberlain MC, Johnston SK (2010) Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neuro-Oncol 96:259–269
Tsimberidou AM, Akerley W, Schabel MC, Hong DS, Uehara C et al (2010) Phase I clinical trial of MPC-6827 (Azixa), a microtubule destabilizing agent, in patients with advanced cancer. Mol Cancer Ther 9:3410–3419
Hwu WJ, Akerley WL, Stephenson J, et al (2010) Final report: MPC-6827 is safely combined with temozolomide for the treatment of patients with metastatic melanoma. J Clin Oncol 28: 15 s (Suppl; abstr 8531)
Grossman K, Colman H, Akerley W, Glantz M, Matsuoko Y, Beelen AP et al (2012) Phase I trial of verubulin (MPC-6827) plus carboplatin in patients with relapsed glioblastoma multiforme. Neuro Oncol 110:257–264
Macdonald DR, Cascino TL, Schold SC, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Wen P, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in Neuro-Oncology Working Group. J Clin Oncol 28:1963–1972
van Heeckeren WJ, Bhakta S, Ortiz J, Duerk J, Cooney MM, Dowlati A et al (2006) Promise of new vascular-disrupting agents balanced with cardiac toxicity: Is it time for oncologists to get to know their cardiologists? J Clin Oncol 24:1485–1488
LoRusso PM, Boerner SA, Hunsberger S (2011) Clinical development of vascular disrupting agents: what lessons can we learn from ASA404? J Clin Oncol 29:2952–2955
Wang ES, Pili R, Seshadri M (2012) Modulation of chemotherapeutic efficacy by vascular disrupting agents: optimizing the sequence and schedule. J Clin Oncol 30:760–761
Bottsford-Miller JN, Colleman RL, Sood AK (2012) Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J Clin Oncol 30:4026–4034
Acknowledgments
Xiaoyu Chai and Brenda Kurland for help with statistical analysis and the Fred Hutchinson Research Cancer Center Biostatistics Shared Resource (funding source P30 CA015704) and Alisa Clein for administrative assistance in the preparation of the manuscript. This study was sponsored by Myriad Inc.
Conflict of interest
None.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
ClinicalTrials.gov Identifier: NCT00892931.
Rights and permissions
About this article
Cite this article
Chamberlain, M.C., Grimm, S., Phuphanich, S. et al. A phase 2 trial of verubulin for recurrent glioblastoma: a prospective study by the brain tumor investigational consortium (BTIC). J Neurooncol 118, 335–343 (2014). https://doi.org/10.1007/s11060-014-1437-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1437-y