Abstract
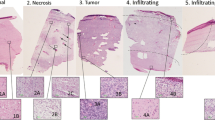
The need exists for a highly accurate, efficient and inexpensive tool to distinguish normal brain tissue from glioblastoma multiforme (GBM) and necrosis boundaries rapidly, in real-time, in the operating room. Raman spectroscopy provides a unique biochemical signature of a tissue type, with the potential to provide intraoperative identification of tumor and necrosis boundaries. We aimed to develop a database of Raman spectra from normal brain, GBM, and necrosis, and a methodology for distinguishing these pathologies. Raman spectroscopy was used to measure 95 regions from 40 frozen tissue sections using 785 nm excitation wavelength. Review of adjacent hematoxylin and eosin sections confirmed histology of each region. Three regions each of normal grey matter, necrosis, and GBM were selected as a training set. Ten regions were selected as a validation set, with a secondary validation set of tissue regions containing freeze artifact. Grey matter contained higher lipid (1061, 1081 cm−1) content, whereas necrosis revealed increased protein and nucleic acid content (1003, 1206, 1239, 1255–1266, 1552 cm−1). GBM fell between these two extremes. Discriminant function analysis showed 99.6, 97.8, and 77.5 % accuracy in distinguishing tissue types in the training, validation, and validation with freeze artifact datasets, respectively. Decreased classification in the freeze artifact group was due to tissue preparation damage. This study shows the potential of Raman spectroscopy to accurately identify normal brain, necrosis, and GBM as a tool to augment pathologic diagnosis. Future work will develop mapped images of diffuse glioma and neoplastic margins toward development of an intraoperative surgical tool.



Similar content being viewed by others
References
Sanai N, Polley M-Y, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8
Krafft C, Sobottka SB, Schackert G, Salzer R (2006) Raman and infrared spectroscopic mapping of human primary intracranial tumors: a comparative study. J Raman Spectrosc 37:367–375
Pohling C, Buckup T, Pagenstecher A, Motzkus M (2011) Chemoselective imaging of mouse brain tissue via multiplex CARS microscopy. Biomed Opt Express 2:2110–2116
Zhou Y, Liu CH, Sun Y, Pu Y, Boydston-White S, Liu Y, Alfano RR (2012) Human brain cancer studied by resonance Raman spectroscopy. J Biomed Opt 17:116021
Mizuno A, Kitajima H, Kawauchi K, Muraishi S, Ozaki Y (1994) Near-infrared Fourier transform Raman spectroscopic study of human brain tissues and tumours. J Raman Spectrosc 25:25–29
Mizuno A, Hayashi T, Tashibu K, Maraishi S, Kawauchi K, Ozaki Y (1992) Near-infrared FT-Raman spectra of the rat brain tissues. Neurosci Lett 141:47–52
Amharref N, Beljebbar A, Dukic S, Venteo L, Schneider L, Pluot M, Manfait M (2007) Discriminating healthy from tumor and necrosis tissue in rat brain tissue samples by Raman spectral imaging. Biochim Biophys Acta 1768:2605–2615
Beljebbar A, Dukic S, Amharref N, Manfait M (2010) Ex vivo and in vivo diagnosis of C6 glioblastoma development by Raman spectroscopy coupled to a microprobe. Anal Bioanal Chem 398:477–487
Koljenovic S, L-Pi Choo-Smith, Bakker Schut TC, Kros JM, van den Berge HJ, Puppels GJ (2002) Discriminating vital tumor from necrotic tissue in human glioblastoma tissue samples by Raman spectroscopy. Lab Invest 82:1265–1277
Krafft C, Sobottka SB, Schackert G, Salzer R (2005) Near infrared Raman spectroscopic mapping of native brain tissue and intracranial tumors. Analyst 130:1070–1077
Koljenovic S, Schut TB, Vincent A, Kros JM, Puppels GJ (2005) Detection of meningioma in dura mater by Raman spectroscopy. Anal Chem 77:7958–7965
Krafft C, Miljanic S, Sobottka SB, Schackert G, Salzer R (2003) Near infrared Raman spectroscopy to study the composition of human brain tissue and tumors. In: Wagnières G (ed) Diagnostic optical spectroscopy in biomedicine II: Proceedings of SPIE, vol 5141. SPIE, Munich, pp 5140–5230
Krafft C, Kirsch M, Beleites C, Schackert G, Salzer R (2007) Methodology for fiber-optic Raman mapping and FTIR imaging of metastases in mouse brains. Anal Bioanal Chem 389:1133–1142
Kirsch M, Schackert G, Salzer R, Krafft C (2010) Raman spectroscopic imaging for in vivo detection of cerebral brain metastases. Anal Bioanal Chem 398:1707–1713
Beleites C, Geiger K, Kirsch M, Sobottka SB, Schackert G, Salzer R (2011) Raman spectroscopic grading of astrocytoma tissues: using soft reference information. Anal Bioanal Chem 400:2801–2816
Bergner N, Bocklitz T, Romeike BFM, Reichart R, Kalff R, Krafft C, Popp J (2012) Identification of primary tumors of brain metastases by Raman imaging and support vector machines. Chemom Intell Lab Syst 117:224–232
Meyer T, Bergner N, Bielecki C, Krafft C, Akimov D, Romeike BF, Reichart R, Kalff R, Dietzek B, Popp J (2011) Nonlinear microscopy, infrared, and Raman microspectroscopy for brain tumor analysis. J Biomed Opt 16:021113
Leslie DG, Kast RE, Poulik JM, Rabah R, Sood S, Auner GW, Klein MD (2012) Identification of pediatric brain neoplasms using Raman spectroscopy. Pediatr Neurosurg 48:109–117
Auner AW, Kast RE, Rabah R, Poulik JM, Klein MD (2013) Conclusions and data analysis: a 6-year study of Raman spectroscopy of solid tumors at a major pediatric institute. Pediatr Surg Int 29:129–140
Bergner N, Krafft C, Geiger KD, Kirsch M, Schackert G, Popp J (2012) Unsupervised unmixing of Raman microspectroscopic images for morphochemical analysis of non-dried brain tumor specimens. Anal Bioanal Chem 403:719–725
Tay L-L, Tremblay RG, Hulse J, Zurakowski B, Thompson M, Bani-Yaghoub M (2011) Detection of acute brain injury by Raman spectral signature. Analyst 136:1620–1626
Ong CW, Shen ZX, He Y, Lee T, Tang SH (1999) Raman microspectroscopy of the brain tissues in the substantia nigra and MPTP-induced Parkinson’s disease. J Raman Spectrosc 30:91–96
Chen P, Shen A, Zhao W, Baek S-J, Yuan H, Hu J (2009) Raman signature from brain hippocampus could aid Alzheimer’s disease diagnosis. Appl Opt 48:4743–4748
Jyothi Lakshmi R, Kartha VB, Murali Krishna C, Solomon RJG, Ullas G, Uma Devi P (2002) Tissue Raman spectroscopy for the study of radiation damage: brain irradiation of mice. Radiat Res 157:175–182
Wills H, Kast R, Stewart C, Rabah R, Pandya A, Poulik J, Auner G, Klein MD (2009) Raman spectroscopy detects and distinguishes neuroblastoma and related tissues in fresh and (banked) frozen specimens. J Pediatr Surg 44:386–391
Bergner N, Romeike BFM, Reichart R, Kalff R, Krafft C, Popp J (2011) Raman and FTIR microspectroscopy for detection of brain metastasis. In: Ramanujam N, Popp J (eds) Clinical and biomedical spectroscopy and imaging II: Proceedings of SPIE, vol 8087. SPIE, Bellingham, p 8087
Krafft C, Bergner N, Romeike B, Reichart R, Kalff R, Geiger K, Kirsch M, Schackert G, Popp J (2012) Raman spectroscopic imaging as complementary tool for histopathologic assessment of brain tumors. In: Kollias N (ed) Photonic therapeutics and diagnostics VIII: Proceedings of SPIE, vol 8207. SPIE, Bellingham, p 8207F
Klecka WR (1980) Discriminant analysis. Sage Publications, Newberry Park
Phillips GR, Harris JM (1990) Polynomial filters for data sets with outlying or missing observations: application to charge-coupled-device-detected Raman spectra contaminated by cosmic rays. Anal Chem 62:2351–2357
Mazet V, Carteret C, Brie D, Idier J, Humbert B (2005) Background removal from spectra by designing and minimising a non-quadratic cost function. Chemom Intell Lab Syst 76:121–133
Eilers PHC (2003) A perfect smoother. Anal Chem 75:3631–3636
Koljenovic S, Schut TCB, Wolthuis R, Vincent AJPE, Hendriks-Hagevi G, Santos L, Kros JM, Puppels GJ (2007) Raman spectroscopic characterization of porcine brain tissue using a single fiber-optic probe. Anal Chem 79:557–564
Socrates G (2001) Infrared and Raman characteristic group frequencies. Wiley, Chichester
Kohler M, Machill S, Salzer R, Krafft C (2009) Characterization of lipid extracts from brain tissue and tumors using Raman spectroscopy and mass spectrometry. Anal Bioanal Chem 393:1513–1520
Movasaghi Z, Rehman S, Rehman IU (2007) Raman spectroscopy of biological tissues. Appl Spectrosc Rev 42:493–541
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalkanis, S.N., Kast, R.E., Rosenblum, M.L. et al. Raman spectroscopy to distinguish grey matter, necrosis, and glioblastoma multiforme in frozen tissue sections. J Neurooncol 116, 477–485 (2014). https://doi.org/10.1007/s11060-013-1326-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1326-9