Abstract
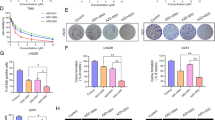
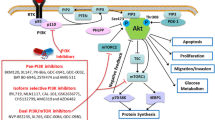
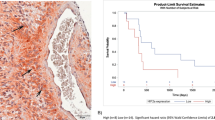
Glioblastoma (GBM) is the most common brain cancer and is highly lethal in both adults and children. 2-methoxyestradiol (2ME2) is a microtubule inhibitor that potently inhibits HIF1α, GBM angiogenesis and tumor growth in preclinical models. In patients, 2ME2 exhibits low toxicity and promising but inconsistent efficacy. Given its preclinical potency and its tolerability in patients, we sought to determine whether 2ME2 therapy could be enhanced by addressing resistance via combination therapy, and with biomarkers to identify responsive glioma subgroups. We demonstrate that the PTEN–PI3K axis regulates HIF1α in glioma models. We utilized isogenic-pairs of glioma cell lines, deficient in PTEN or stably reconstituted with PTEN, to determine the role of PTEN in 2ME2 sensitivity in vitro and in vivo. Chou–Talalay synergy studies reveal significant synergy when a pan-PI3K inhibitor is combined with 2ME2. This synergistic activity was correlated with a synergistic suppression of HIF1α accumulation under hypoxic conditions in glioma models. In vivo, 2ME2 markedly inhibited tumor-induced angiogenesis and significantly reduced tumor growth only in a PTEN reconstituted GBM models in both subcutaneous and orthotopic intracranial mouse models. Collectively, these results: (1) suggest that PTEN status predicts sensitivity to 2ME2 and (2) justify exploration of 2ME2 combined with pan-PI3K inhibitors for the treatment of this intractable brain cancer.





Similar content being viewed by others
References
Buckner JC (2003) Factors influencing survival in high-grade gliomas. Semin Oncol 30(6 Suppl 19):10–14
DeAngelis LM (2001) Brain tumors. N Engl J Med 344(2):114–123
Srividya MR et al (2011) Homozygous 10q23/PTEN deletion and its impact on outcome in glioblastoma: a prospective translational study on a uniformly treated cohort of adult patients. Neuropathology 31(4):376–383
Stupp R et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Cancer Genome Atlas Research Network (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455(7216):1061–1068
Gallia GL et al (2006) PIK3CA gene mutations in pediatric and adult glioblastoma multiforme. Mol Cancer Res 4(10):709–714
Scheid MP, Woodgett JR (2003) Unravelling the activation mechanisms of protein kinase B/Akt. FEBS Lett 546(1):108–112
Castellino RC, Muh CR, Durden DL (2009) PI-3 kinase-PTEN signaling node: an intercept point for the control of angiogenesis. Curr Pharm Des 15(4):380–388
Sun H et al (1999) PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci USA 96(11):6199–6204
Wen S et al (2001) PTEN controls tumor-induced angiogenesis. PNAS 98(8):4622–4627
Forsythe JA et al (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16(9):4604–4613
Ivan M et al (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292(5516):464–468
Jaakkola P et al (2001) Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292(5516):468–472
Semenza G (2002) Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol 64(5–6):993–998
Jiang BH et al (2001) Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ 12(7):363–369
Zhong H et al (2000) Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res 60(6):1541–1545
Zundel W et al (2000) Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev 14(4):391–396
Alvarez-Tejado M et al (2002) Lack of evidence for the involvement of the phosphoinositide 3-kinase/Akt pathway in the activation of hypoxia-inducible factors by low oxygen tension. J Biol Chem 277(16):13508–13517
Arsham AM et al (2002) Phosphatidylinositol 3-kinase/Akt signaling is neither required for hypoxic stabilization of HIF-1 alpha nor sufficient for HIF-1-dependent target gene transcription. J Biol Chem 277(17):15162–15170
Mabjeesh NJ et al (2003) 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3(4):363–375
Kirches E, Warich-Kirches M (2009) 2-methoxyestradiol as a potential cytostatic drug in gliomas? Anticancer Agents Med Chem 9(1):55–65
Kang SH et al (2006) Antitumor effect of 2-methoxyestradiol in a rat orthotopic brain tumor model. Cancer Res 66(24):11991–11997
Lis A et al (2004) 2-Methoxyestradiol inhibits proliferation of normal and neoplastic glial cells, and induces cell death, in vitro. Cancer Lett 213:57–65
James J et al (2007) Phase I safety, pharmacokinetic and pharmacodynamic studies of 2-methoxyestradiol alone or in combination with docetaxel in patients with locally recurrent or metastatic breast cancer. Invest New Drugs 25(1):41–48
Bruce JY et al (2012) A phase II study of 2-methoxyestradiol nanocrystal colloidal dispersion alone and in combination with sunitinib malate in patients with metastatic renal cell carcinoma progressing on sunitinib malate. Invest New Drugs 30(2):794–802
Sweeney C et al (2005) A phase II multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin Cancer Res 11(18):6625–6633
ClinicalTrials.gov. Phase 2 study of panzem nanocrystal colloidal dispersion (NCD) in combination with fixed-dose temozolomide to patients with recurrent glioblastoma multiforme (GBM). http://clinicaltrials.gov/ct2/show/NCT00481455
ClinicalTrials.gov. A phase 2 study with panzem in patients with relapsed or plateau phase multiple myeloma. http://clinicaltrials.gov/ct2/show/NCT00592579
ClinicalTrials.gov. A combination study to determine the safety and efficacy of panzem NCD with avastin in metastatic carcinoid tumors. http://clinicaltrials.gov/ct2/show/NCT00328497
Dahut WL et al (2006) Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol Ther 5(1):22–27
Mooberry SL (2003) New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Curr Opin Oncol 15(6):425–430
Duman BB et al (2013) PTEN, Akt, MAPK, p53 and p95 expression to predict trastuzumab resistance in HER2 positive breast cancer. J BUON 18(1):44–50
Sami A, Karsy M (2013) Targeting the PI3K/AKT/mTOR signaling pathway in glioblastoma: novel therapeutic agents and advances in understanding. Tumour Biol 34(4):1991–2002
Wischhusen J et al (2003) CP-31398, a novel p53-stabilizing agent, induces p53-dependent and p53-independent glioma cell death. Oncogene 22(51):8233–8245
Garlich JR et al (2008) A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res 68(1):206–215
Rong Y et al (2005) PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res 65(4):1406–1413
Andrews NC, Faller DV (1991) A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res 19(9):2499
Peirce SK et al (2011) The PI-3 kinase-Akt-MDM2-survivin signaling axis in high-risk neuroblastoma: a target for PI-3 kinase inhibitor intervention. Cancer Chemother Pharmacol 68(2):325–335
Mabjeesh NJ et al (2003) 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell 3:363–375
Ricker JL et al (2004) 2-Methoxyestradiol inhibits hypoxia-inducible factor 1α, tumor growth, and angiogenesis and augments paclitaxel efficacy in head and neck squamous cell carcinoma. Clin Cancer Res 10:8665–8673
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Su JD et al (2003) PTEN and phosphatidylinositol 3′-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Cancer Res 63(13):3585–3592
Said R et al (2013) P53 mutations in advanced cancers: clinical characteristics, outcomes, and correlation between progression-free survival and bevacizumab-containing therapy. Oncotarget 4(5):705–714
Sermeus A, Michiels C (2011) Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis 2:e164
Chen D et al (2003) Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function. J Biol Chem 278(16):13595–13598
Stambolic V et al (2001) Regulation of PTEN transcription by p53. Mol Cell 8(2):317–325
Mellinghoff IK et al (2005) Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med 353(19):2012–2024
Acknowledgments
We thank Dr. H.K Shu for providing the EGFRvIII transduced LN229 cell line. This work was supported in part by a grant from EntreMed, Inc. 9640 Medical Center Drive, Rockville, MD 20850 and NIH grants CA94233 and HL091365 to Dr. Donald L. Durden, and a grant from the Musella Foundation and Heroes of Hope Coalition to Dr. Carrie Muh.
Conflict of interest
D. Durden discloses financial conflict of interest related to the development of SF1126. This aspect has been reviewed by the UCSD committee on conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muh, C.R., Joshi, S., Singh, A.R. et al. PTEN status mediates 2ME2 anti-tumor efficacy in preclinical glioblastoma models: role of HIF1α suppression. J Neurooncol 116, 89–97 (2014). https://doi.org/10.1007/s11060-013-1283-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1283-3