Abstract
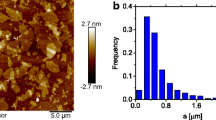
Graphene and graphene oxide are potential candidates as nanofluids for thermal management applications. Here, we investigate the rheological properties and intrinsic viscosity of aqueous suspension of graphene and use the measured intrinsic viscosity to determine the aspect ratio of graphene oxide. Dilute suspension of graphene oxide (0.05 to 0.5 mg/mL) exhibits a shear thinning behavior at low shear rates followed by a shear-independent region that starts at shear rate between 5 and 100/s depending on the concentration. This shear thinning behavior becomes more pronounced with the increase of particle loading. Moreover, AFM imaging of the dried graphene oxide indicates the evolution of irregular and thin low fractal aggregates of 0.3–1.8 nm thickness at lower concentrations to oblate compact structures of 1–18 nm thickness of nanosheets at higher concentration. These observations elucidate the microstructure growth mechanisms of graphene oxide in multiphase systems, which are important for nanofluids applications and for dispersing graphene and graphene oxide in composite materials. The suspension has a very high intrinsic viscosity of 1661 due to the high graphene oxide aspect ratio. Based on this intrinsic viscosity, we predict graphene oxide aspect ratio of 2445. While the classical Einstein and Batchelor models underestimate the relative viscosity of graphene oxide suspension, Krieger–Dougherty prediction is in a good agreement with the experimental measurement.





Similar content being viewed by others
References
Anbia M, Hariri SA et al (2010) Adsorptive removal of anionic dyes by modified nanoporous silica SBA-3. Appl Surf Sci 256(10):3228–3233
Azizian S, Haerifar M et al (2009) Adsorption of methyl violet onto granular activated carbon: equilibrium, kinetics and modeling. Chem Eng J 146(1):36–41
Batchelor G (1977) The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J Fluid Mech 83(01):97–117
Boluk Y, Lahiji R et al (2011) Suspension viscosities and shape parameter of cellulose nanocrystals (CNC). Colloids Surf A 377(1):297–303
Cai W, Moore AL et al (2010) Thermal transport in suspended and supported monolayer graphene grown by chemical vapor deposition. Nano Lett 10(5):1645–1651
Chen H, Ding Y et al (2007) Rheological behaviour of nanofluids. New J Phys 9(10):367
Chen S, Zhang J et al (2010) Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 252(1–3):149–156
Coleman JN, Khan U et al (2006) Small but strong: a review of the mechanical properties of carbon nanotube–polymer composites. Carbon 44(9):1624–1652
Deng F, Zheng Q-S et al (2007) Effects of anisotropy, aspect ratio, and nonstraightness of carbon nanotubes on thermal conductivity of carbon nanotube composites. Appl Phys Lett 90(2):021914-021914-021913
Einstein A (1906) A new determination of molecular dimensions. Ann Phys 19(2):289–306
Gong JL, Wang B et al (2009) Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as adsorbent. J Hazard Mater 164(2–3):1517–1522
Grande L, Chundi VT et al (2012) Graphene for energy harvesting/storage devices and printed electronics. Particuology 10(1):1–8
Gupta VK, Suhas (2009) Application of low-cost adsorbents for dye removal—a review. J Environ Manag 90(8):2313–2342. http://www.sciencedirect.com/science/article/pii/S0301479708003290#
Güven N (1992) Molecular aspects of clay-water interactions. Clay-water interface and its rheological implications 4:2–79
Iwamoto S, Lee S-H, et al (2013) Relationship between aspect ratio and suspension viscosity of wood cellulose nanofibers. Polym J. doi:10.1038/pj.2013.64
Jiang B, Liu C et al (2007) The effect of non-symmetric distribution of fiber orientation and aspect ratio on elastic properties of composites. Compos B Eng 38(1):24–34
Karagöz S, Tay T et al (2008) Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresour Technol 99(14):6214–6222
Kim H, Abdala AA et al (2010) Graphene/polymer nanocomposites. Macromolecules 43(16):6515–6530
Krieger IM, Dougherty TJ (1959) A mechanism for non-Newtonian flow in suspensions of rigid spheres. J Rheol 3:137
Kuhn W, Kuhn H (1945) Die Abhängigkeit der Viskosität vom Strömungsgefälle bei hochverdünnten Suspensionen und Lösungen. Helv Chim Acta 28(1):97–127
Lee C-L, Chen C-H et al (2013a) Graphene nanosheets as ink particles for inkjet printing on flexible board. Chem Eng J 230:296–302
Lee SW, Kim KM et al (2013b) Study on flow boiling critical heat flux enhancement of graphene oxide/water nanofluid. Int J Heat Mass Transf 65:348–356
Lerf A, He H et al (1998) Structure of graphite oxide revisited. J Phys Chem B 102(23):4477–4482
Liu P, Zhang L (2007) Adsorption of dyes from aqueous solutions or suspensions with clay nano-adsorbents. Sep Purif Technol 58(1):32–39
Liu Z, Zhou A et al (2009) Adsorption behavior of methyl orange onto modified ultrafine coal powder. Chin J Chem Eng 17(6):942–948
Ma W, Yang F et al (2013) Silicone based nanofluids containing functionalized graphene nanosheets. Colloids Surf A 431:120–126
Novoselov K, Geim A et al (2004) Electric field effect in atomically thin carbon films. Science 306(5696):666
Parra-Vasquez ANG, Stepanek I et al (2007) Simple length determination of single-walled carbon nanotubes by viscosity measurements in dilute suspensions. Macromolecules 40(11):4043–4047
Potts JR, Dreyer DR et al (2011) Graphene-based polymer nanocomposites. Polymer 52(1):5–25
Prud’homme RK, Aksay IA, et al (2008) Conductive ink containing thermally exfoliated graphite oxide and method of making a conductive circuit using the same, US patent App. 20,080/302,561
Rafatullah M, Sulaiman O et al (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177(1–3):70–80
Teng M-Y, Lin S-H (2006) Removal of methyl orange dye from water onto raw and acid activated montmorillonite in fixed beds. Desalination 201(1–3):71–81
Yao Y, Xu F et al (2010) Adsorption behavior of methylene blue on carbon nanotubes. Bioresour Technol 101(9):3040–3046
Yu W, Xie H et al (2011) Significant thermal conductivity enhancement for nanofluids containing graphene nanosheets. Phys Lett A 375(10):1323–1328
Acknowledgments
The authors acknowledge Dr. Tewfik Souier for his assistance with AFM imaging.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tesfai, W., Singh, P., Shatilla, Y. et al. Rheology and microstructure of dilute graphene oxide suspension. J Nanopart Res 15, 1989 (2013). https://doi.org/10.1007/s11051-013-1989-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-013-1989-3