Abstract
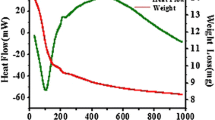
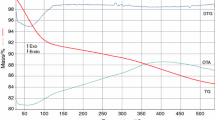
We report a novel technique for the formation of metal nanoparticles, based on electrolysis of the alcogels containing metal chlorides. The alcogel was formed from TEOS, water, ethanol, and nickel chloride, and subjected to galvanostatic electrolysis. This resulted in successful formation of Ni nanoparticles inside the silica gel. Average particle size of FCC Ni lies between 18 and 20 nm. The formation of tetragonal nickel (a sub-oxide of nickel) as well as NiO were also detected by XRD and SAED. The resistivity measurements showed that the nickel nanoparticles were separated from each other by Ni(O) present between them. Magnetic studies based on ZFC and FC measurements below room temperature (up to 5 K) and above room temperature (up to 700 K) were conducted using SQUID and Magnetic TGA, respectively, which showed strong magnetic irreversibility as attributable to exchange interaction between metallic and oxide phases and mutual interactions among metallic particles in the network structure. The blocking temperature (~600 K) of the samples was above room temperature. M–H studies based on VSM showed an increase in magnetic coercivity with the formation of NiO. A magnetic transition associated with tetragonal nickel was seen at 10 K.







Similar content being viewed by others
References
Boiadjieva T, Cappelletti G, Ardizzone S, Rondinini S, Vertova A (2003) Nanocrystalline titanium oxide by sol-gel method: the role of the solvent removal step. Phys Chem Chem Phys 5:1689–1694
Brinker CJ, Scherer GW (1989) Sol-gel science: the physics and chemistry of sol-gel processing. Academic Press, California, USA
Cai WP, Zhang LD (1997) Synthesis and structural and optical properties of mesoporous silica containing silver nanoparticles. J Phys: Condens Matter 9:7257–7267
Chipara M, Hui D, Sankar J, Leslie-Pelecky D, Bender A, Yue L, Skomski R, Sellmyer DJ (2004) On styrene-butadiene-styrene-barium ferrite nanocomposites. Composites B 35:235–243
Cintora-Gonzalez O, Estournes C, Richard-Plouet M, Guille JL (2001) Nickel nano-particles in silica gel monoliths: control of the size and magnetic properties. Mater Sci Eng C 15:179–182
Dorman JL, Fiorani D (eds) (1992) Magnetic properties of fine particles. North Holland, Amsterdam
Ennas G, Mei A, Musinu A, Piccaluga G, Pinna G, Solinas S (1998) Sol-gel preparation and characterization of Ni-SiO2 nanocomposites. J Noncryst Solids 232:587–593
Ennas G, Falqui A, Marras S, Sangregorio C, Marongiu G (2004) Influence of metal content on size, dispersion, and magnetic properties of iron-cobalt alloy nanoparticles embedded in silica matrix. Chem Mater 16:5659–5663
Estournes C, Lutz T, Happich J, Quaranta T, Wissler P, Guille JL (1997) Nickel nanoparticles in silica gel: preparation and magnetic properties. J Magn Magn Mater 173:83–92
Fidalgo A, Ilharco LM (2005) The influence of the wet gels processing on the structure and properties of silica xerogels. Microporous Mesoporous Mater 84:229–235
Jamal EMA, Joy PA, Kurian P, Anantharamana MR (2009) Synthesis of nickel-rubber nanocomposites and evaluation of their dielectric properties. Mater Sci Eng B 156:24–31
Kan CX, Cai WP, Li ZS, Fu GH, Zhang LD (2003) Reduction effect of pore wall and formation of Au nanowires inside monolithic mesoporous silica. Chem Phys Lett 382:318–324
Liu W, Zhong W, Jiang HY, Tang NJ, Wu XL, Du AY (2005) Synthesis and magnetic properties of FeNi3/Al2O3 core-shell nanocomposites. Eur Phys J B 46:471–474
Liu W, Zhong W, Jiang HY, Tang NJ, Wu XL, Du YW (2006) Highly stable alumina-coated iron nanocomposites synthesized by wet chemistry method. Surf Coat Technol 200:5170–5174
Ma X, Zhang Y, Ge S, Zhang Z, Yan D, Xiao DT (2009) Thick film nanoparticulate composites and method of manufacture thereof. US Patent 7,485,366 B2
Moriarty P (2001) Nanostructured materials. Rep Prog Phys 64:297–381
Nayak BB, Vitta S, Nigum AK, Bahadur D (2005) Transport and magnetic properties of encapsulated Ni–Ni–O/Zr–O nanostructures. IEEE Trans Magn 41:3298–3300
Ohldag H, Scholl A, Nolting F, Anders S, Hillebrecht FU, Stöhr J (2001) Spin reorientation at the antiferromagnetic NiO(001) surface in response to an adjacent ferromagnet. Phys Rev Lett 86:2878–2881
Peng K, Zhou LP, Hu A, Tang YH, Li D (2008) Synthesis and magnetic properties of Ni–SiO2 nanocomposites. Mater Chem Phys 111:34–37
Polshettiwar V, Molnár Á (2007) Silica-supported Pd catalysts for Heck coupling reactions. Tetrahedron 63:6949–6976
Roy A, Srinivas V, Ram S, De Toro JA, Mizutani U (2005) Structure and magnetic properties of oxygen-stabilized tetragonal Ni nanoparticles prepared by borohydride reduction method. Phys Rev B 71:184443
Roy A, Srinivas V, De Toro JA, Goff JP (2006) Low-temperature magnetization dynamics of oxygen-stabilized tetragonal Ni nanoparticles. Phys Rev B 74:104402
Skumryev V, Stoyanov S, Zhang Y, Hadjipanayis G, Givord D, Nogues J (2003) Beating the superparamagnetic limit with exchange bias. Nature 423:850–853
Sun XC, Dong XL (2002) Magnetic properties and microstructure of carbon encapsulated Ni nanoparticles and pure Ni nanoparticles coated with NiO layer. Mater Res Bull 37:991–1004
Tang NJ, Jiang HY, Zhong W, Wu XL, Zou WQ, Du YW (2006) Synthesis and magnetic properties of Fe/SiO2 nanocomposites prepared by a sol-gel method combined with hydrogen reduction. J Alloy Compd 419:145–148
Tom RT, Nair AS, Singh N, Aslam M, Nagendra CL, Philip R, Vijayamohanan K, Pradeep T (2003) Freely dispersible Au@TiO2, Au@ZrO2, Ag@TiO2, and Ag@ZrO2 core-shell nanoparticles: one-step synthesis, characterization, spectroscopy, and optical limiting properties. Langmuir 19:3439–3445
van Lierop J, Lewis LH, Williams KE, Gambino RJ (2002) Magnetic exchange effects in a nanocomposite Ni/NiO film. J Appl Phys 91:7233–7235
Wu YC, Zhang L, Li GH, Liang CH, Huang XM, Zhang Y, Song GM, Jia JH, Chen ZX (2001) Synthesis and characterization of nanocomposites with palladium embedded in mesoporous silica. Mater Res Bull 36:253–263
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rana, M.Z., Mehmood, M., Ahmad, J. et al. Synthesis of nickel nanoparticles in silica by alcogel electrolysis. J Nanopart Res 13, 375–384 (2011). https://doi.org/10.1007/s11051-010-0040-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-010-0040-1