Abstract
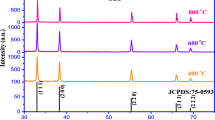
Surface-capped CdSe and CdTe nano-crystals (NCs) have been synthesized using cadmium acetate, oleic acid and respective tri-octylphosphine chalcogenide (TOPE; E = Se/Te) in diphenyl ether (DPE). Well-dispersed CdSe particles showed two absorption bands at the region of 431–34 and 458–60 nm in optical absorption study. A band-edge emission resulted at 515 nm with an excitation energy of 400 nm, in its photoluminescence (PL) spectrum. Similarly, UV–visible absorption study of CdTe revealed an absorption band at <700 nm. The broadened X-ray diffraction (XRD) pattern showed that at higher reaction temperature cubic CdSe but hexagonal CdTe can be obtained with crystallite size of <10 nm. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) revealed that agglomerated particles are of spherical nature. The inter-planar spacing in CdTe was measured to be 0.406 nm, a characteristic of (100) lattice plane in hexagonal CdTe. X-ray photoelectron spectroscopy (XPS) showed that CdSe NCs have better air stability stable than CdTe. Presence of organic moiety around the semiconductor particles was confirmed by infra-red (IR) spectroscopy.













Similar content being viewed by others
References
Afzaal M, Crouch D, Malik MA, Motevalli M, O’Brien P, Park J-H (2003) Deposition of CdSe thin films using a novel single-source precursor; [MeCd{(SePiPr2)2N}]2. J Mater Chem 13:639–640
Alivisatos AP (1998) Electrical studies of semiconductor-nanocrystals colloids. MRS Bull February:18–23
Briggs D, Seah MP (1996) Particle surface analysis. In: Auger and X-ray photoelectron spectroscopy, vol 1, 2nd edn. Wiley, Chichester
Chen Y, Gao L (2002) Synthesis and characterization of pyramidal CdSe nanoparticles. Chem Lett 6:556–557. doi:10.1246/cl.2002.556
Chu M, Liu G (2006) Synthesis of liposomes-templated CdSe hollow and solid nanospheres. Mater Lett 60:11–14. doi:10.1016/j.matlet.2005.07.086
Colvin VL, Schlamp MC, Alivisatos AP (1994) Light emitting diodes made from cadmium selenides nanocrystals and a semiconducting polymer. Nature 370:354–358. doi:10.1038/370354a0
Deng Z-X, Li L, Li Y (2003) Novel inorganic-organic layered structures: crystallographic understanding of both phase & morphology formations of one-dimensional CdE (E = S, Se, Te) nanorods in ethylenediamine. Inorg Chem 42:2331–2341. doi:10.1021/ic025846d
Diao X-L, Xia Y-S, Zhang T-L, Li Y, Zhu C-Q (2007) Fluorescence-detecting cationic surfactants using luminescent CdTe quantum dots as probes. Anal Bioanal Chem 388:1191–1197. doi:10.1007/s00216-007-1319-7
Firth AV, Haggata SW, Khanna PK, Williams SJ, Allen JW, Magennis SW, Samuel IDW, Cole-Hamilton DJ (2004) Production and luminescent properties of CdSe and CdS nanoparticle-polymer composites. J Lumin 109:163–173
Hambrock J, Birkner A, Fischer RA (2001) Synthesis of CdSe nanoparticles using various organometallic cadmium precursors. J Mater Chem 11:3197–3201. doi:10.1039/b104231a
Han H-y, Sheng Z-h, Liang J-g (2006) A novel method for the preparation of water-soluble and small-size CdSe quantum dots. Mater Lett 60:3782–3785
Khanna PK (2008) Near band-gap emission from CdSe nano-crystals synthesized from direct reaction of cadmium acetate and octeno-1,2,3-selenadiazole. J Synth React Inorg Met-Org Nano-Met Chem 38(5):409–413
Khanna PK, Gorte RM, Gokhale R (2004a) Synthesis of cadmium selenide from hepteno-1,2,3-selenadiazol and cadmium salts in ethylene glycol. Mater Lett 58:966–969. doi:10.1016/j.matlet.2003.07.035
Khanna PK, Morley CP, Gorte RM, Gokhale R, Subbarao VVVS, Satyanarayana CVV (2004b) Simple and effective synthesis of cadmium selenide in aqueous N,N′ dimethylformamide. Mater Chem Phys 83:323–327. doi:10.1016/j.matchemphys.2003.10.013
Lee HJ, Kim D-Y, Yoo J-S, Bang J, Kim S, Park S-M (2007) Anchoring cadmium chalcogenide quantum dots (QDs) onto stable oxide; semiconductors for QD sensitized solar cells. Bull Korean Chem Soc 28:953–958
Liu L, Peng Q, Li Y (2008) Preparation of CdSe QDs with full colour emission based on a room temperature injection technique. Inorg Chem 47(11):5022–5028
Murray CB, Norris DJ, Bawendi MG (1993) Synthesis & characterization of nearly monodisperse CdE (E = S, Se, Te) semiconductor nanocrystallites. J Am Chem Soc 115:8706–8715. doi:10.1021/ja00072a025
Murray CB, Sun S, Gaschler W, Doyle H, Betley TA, Kagan CR (2001) Colloidal synthesis of nanocrystals and nanocrystals superlattices. IBM J Res Dev 45:47–55
Nie W, An L, Jiang B, Ji X-L (2004) A facile synthesis of CdSe and CdTe nanorods assisted by myristic acid. Chem Lett 7(33):836–837. doi:10.1246/cl.2004.836
Pei J-H, Lin CM, Chuu D-S (1998) Characterization of CdTe film grown on a Si (111) substrate. Chin J Physiol 1(36):44–52
Peng ZA, Peng X (2001) Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. J Am Chem Soc 123:183–184. doi:10.1021/ja003633m
Protasenko V, Bacinello D, Kuno M (2006) Experimental determination of the absorption cross-section and molar extinction coefficient of CdSe and CdTe nanowires. J Phys Chem B 110(50):25322–25331
Qu L, Yu WW, Peng X (2004) In situ observation of the nucleation & growth of CdSe nanocrystals. Nano Lett 4:465–469. doi:10.1021/nl035211r
Raevskaya AE, Stroyuk AL, Kuchmiy SYa, Azhniuk YuM, Dzhagan VM, Yukhymchuk VO, Valakh MYa (2006) Growth and spectroscopic characterization of CdSe nanoparticles synthesized from CdCl2 and Na2SeSO3 in aqueous gelatine solutions. Colloids Surf A Physicochem Eng Asp 290:304–309
Rosenthal SJ, McBride J, Pennycook SJ, Feldman LC (2007) Synthesis, surface studies, composition and structural characterization of CdSe, core/shell and biologically active nanocrystals. Surf Sci Rep 62:111–157. doi:10.1016/j.surfrep.2007.02.001
Sashchiuk A, Amirav L, Bashouti M, Krueger M, Sivan U, Lifshitz E (2004) PbSe nanocrystals assemblies: synthesis and structural, optical and electrical characterization. Nano Lett 4:159–165. doi:10.1021/nl0345116
Trindale T, Monteiro OC, O’Brien P, Motevalli M (1999) Synthesis of PbSe nanocrystallites using a single-source method. The X-ray crystal structure of lead(II) diethyldiselenocarbamate. Polyhedron 18:1171–1175. doi:10.1016/S0277-5387(98)00411-2
Wang Q, Seo D-K (2006) Synthesis of deep-red-emitting CdSe quantum dots and general non-inverse-square behavior of quantum confinement in CdSe quantum dots. Chem Mater 18:5764–5767
Wu N, Fu L, Su M, Aslam M, Wong KC, Dravid VP (2004) Interaction of fatty acid monolayers with cobalt nanoparticles. Nano Lett 4:383
Yen BKH, Stott NE, Jensen KF, Bawendi MG (2003) A continuous-flow microcapillary reactor for the preparation of a size series of CdSe nanocrystals. Adv Mater 15:1858–1862. doi:10.1002/adma.200305162
Acknowledgement
PKK thanks Dr. T. L. Prakash, Director, C-MET, Hyderabad, for permitting KRSR to work in his laboratory at Pune and DST (Government of India) for financial support through grant no. SR/S1/PC-17/2006.
Author information
Authors and Affiliations
Corresponding author
Additional information
PKK dedicates this work to his late father-RP Khanna
Rights and permissions
About this article
Cite this article
Khanna, P.K., Srinivasa Rao, K., Patil, K.R. et al. One-pot synthesis of oleic acid-capped cadmium chalcogenides (CdE: E = Se, Te) nano-crystals. J Nanopart Res 12, 101–109 (2010). https://doi.org/10.1007/s11051-008-9581-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-008-9581-y