Abstract
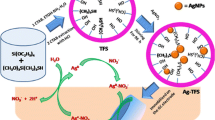
The synthesis of aqueous dispersion of spherical, underivatized silver nanoparticles (Ag-NPs) stabilized by macrocyclic polyammonium chlorides (MCPAC), [28]ane-(NH2 +)6O2·6Cl− (28-MCPAC) and [32]ane-(NH2 +)8·8Cl− (32-MCPAC), which are evidently anion receptors, is reported. As-synthesized Ag-NPs are characterized by UV-vis spectroscopy and transmission electron microscopy (TEM). The 28/32-MCPAC-stabilized Ag-NPs show the surface plasmon band around 400 nm. The TEM-images show that the particles are spherical and well-dispersed. By tuning the 28/32-MCPAC:Ag-OAc (silver acetate) ratio, nanoparticles with different core diameters ranging from 13 to 8 nm for 28-MCPAC and from 10 to 6 nm for 32-MCPAC can be obtained. The advantage of using MCPAC as stabilizers is that they make the particles functionalized for sensing anions. Thus, the potential of the as-synthesized Ag-NPs for sensing phosphates: H2PO4 − (monobasic phosphate, MBP), HPO4 2− (dibasic phosphate, DBP) and PO4 3− (tribasic phosphate, TBP) is investigated spectroscopically. Interaction of phosphate ions with macrocyclic polyammonium cations makes the Ag-NPs bare, leading agglomeration. The phosphate-assisted agglomeration of 32-MCPAC-Ag-NPs follow the order TBP > DBP ≫ MBP.









Similar content being viewed by others
References
Bazzicalupi C, Bencini A, Bianchi A, Cecchi M, Escuder B, Fusi V et al (1999) Thermodynamics of phosphates and pyrophosphates anions binding by polyammonium receptors. J Am Chem Soc 121(29):6807–6815. doi:10.1021/ja983947y
Beer PD, Gale PA (2001) Anion recognition and sensing: a state of the art and future perspectives. Angew Chem Int Ed 40:486–516. doi:10.1002/1521-3773(20010202)40:3<486::AID-ANIE486>3.0.CO;2-P
Bianchi A, Bowman-James K, Garcia-España E (1997) Supramolecular chemistry. Wiley-VCH, New York
Braun E, Erichen Y, Siven U, Yoseph GB (1998) DNA-templated assembly and electrode attachment of a conducting silver wire. Nature 391:775–778. doi:10.1038/35826
Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R (1994) Synthesis of thiol-derivatized gold nanoparticles in a two-phase liquid–liquid system. J Chem Soc Chem Commun 801–802. doi:10.1039/c39940000801
Chen S, Kimura K (1999) Synthesis and characterization of carboxylate-modified gold nanoparticle powders dispersible in water. Langmuir 15(4):1075–1082. doi:10.1021/la9812828
Chen W, Dong S, Wang E (2003) Synthesis and self-assembly of cetyltrimethyl-ammonium bromide-capped gold nanoparticles. Langmuir 19(22):9434–9439. doi:10.1021/la034818k
Elechiguerra JL, Burt JL, Morones JR, Camacho-Bragado A, Gao X, Lara HH et al (2005) Interaction of silver nanoparticles with HIV-I. J Nanobiotechnol 3(6):1–10. doi:10.1186/1477-3155-3-6
Fink J, Kiely CJ, Bethell D, Schiffrin DJ (1998) Self-organization of nanosized gold particles. Chem Mater 10(3):922–926. doi:10.1021/cm970702w
Fitzmaurice D, Rao SN, Preece JA, Stoddart JF, Wenger S, Zaccheroni N (1999) Heterosupramolecular chemistry: programmed pseudorotaxane assembly at the surface of a nanocrystal. Angew Chem Int Ed Engl 38(8):1147–1150. doi:10.1002/(SICI)1521-3773(19990419)38:8<1147::AID-ANIE1147>3.0.CO;2-A
Garcia-España E, Diaz P, Llinares JM, Bianchi A (2006) Anion coordination chemistry in aqueous solution of polyammonium receptors. Coord Chem Rev 250:2952–2986. doi:10.1016/j.ccr.2006.05.018
Hailstone RK (1995) Computer simulation studies of silver cluster formation on AgBr microcrystals. J Phys Chem 99(13):4414–4428. doi:10.1021/j100013a009
Henglein A, Giersig M (1999) Formation of colloidal silver nanoparticles: capping action of citrate. J Phys Chem B 103(44):9533–9539. doi:10.1021/jp9925334
Hussain I, Brust M, Papworth AJ, Cooper AI (2003) Preparation of acrylate-stabilized gold and silver hydrosols and gold-polymer composite films. Langmuir 19(11):4831–4835. doi:10.1021/la020710d
Jain P, Pradeep T (2005) Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol Bioeng 90:59–63. doi:10.1002/bit.20368
Kamat PV (2002) Photophysical, photochemical and photocatalytic aspects of metal nanoparticles. J Phys Chem B 106(32):7729–7744. doi:10.1021/jp0209289
Kang SY, Kim K (1998) Comparative study of dodecanethiol-derivatized silver nanoparticles prepared in one-phase and two-phase systems. Langmuir 14(1):226–230. doi:10.1021/la970696i
Kelly KL, Coronado E, Zhao LI, Schatz GC (2003) The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 107(3):668–677. doi:10.1021/jp026731y
Lee PC, Meisel D (1982) Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem 86(17):3391–3395. doi:10.1021/j100214a025
Li X, Jhang J, Xu W, Jia H, Wang X, Yang B et al (2003) Mercaptoacetic acid-capped silver nanoparticles colloid: formation, morphology, and SERS activity. Langmuir 19(10):4285–4290. doi:10.1021/la0341815
Lin SY, Liu SW, Lin CM, Chen CH (2002) Recognition of potassium ion in water by 15-crown-5 functionalized gold nanoparticles. Anal Chem 74(2):330–335. doi:10.1021/ac0156316
Lin SY, Chen CH, Lin MC, Hsu HF (2005) A cooperative effect of biofunctionalized nanoparticles on recognition: sensing alkali ions by crown and carboxylate moieties in aqueous media. Anal Chem 77(15):4821–4828. doi:10.1021/ac050443r
Liu CY, Chen WH (1998) Electrophoretic separation of inorganic anions with an anion complex one-modified capillary column. J Chromatogr A 815:251–263. doi:10.1016/S0021-9673(98)00481-6
Liu J, Alvarez J, Kaifer AE (2000) Metal nanoparticles with a knack for molecular recognition. Adv Mater 12(18):1381–1383 and references cited therein. doi:10.1002/1521-4095(200009)12:18<1381::AID-ADMA1381>3.0.CO;2-U
Liu C, Yang X, Yuana H, Zhou Z, Xiao D (2007a) Preparation of silver nanoparticles and its application to the determination of ct-DNA. Sensors 7:708–718
Liu CY, Chen TH, Misra TK (2007b) A macrocyclic polyamine as an anion receptor in the capillary electrochromatographic separation of carbohydrates. J Chromatogr A 1154:407–415. doi:10.1016/j.chroma.2007.03.083
Lu Y, Liu GL, Lee LP (2005) High-density silver nanoparticle film with temperature-controllable interparticle spacing for a tunable surface enhanced Raman scattering substrate. Nano Lett 5(1):5–9. doi:10.1021/nl048965u
Maier SA, Brongersma ML, Kik PG, Meltzer S, Requicha AAG, Atwater HA (2001) Plasmonics—a route to nanoscale optical devices. Adv Mater 13(19):1501–1505. doi:10.1002/1521-4095(200110)13:19<1501::AID-ADMA1501>3.0.CO;2-Z
Mayer ABR, Mark JE (2002) Poly(2-hydroxyalkyl methacrylates) as stabilizers for colloidal noble metal nanoparticles. Polymer (Guildf) 41(4):1627–1631. doi:10.1016/S0032-3861(99)00368-7
Misra TK, Liu CY (2008) Synthesis of 28-membered macrocyclic polyammonium cations functionalized gold nanoparticles and their potential for sensing nucleotides. J Colloid Interface Sci (in press). doi:10.1016/j/jcis.2008.06.056
Misra TK, Chen TS, Wong KT, Liu CY (2005) A convenient modified short route for the preparation of [32]ane-N8 hydrochloride. J Chin Chem Soc 52(4):793–797
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT et al (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16(10):2346–2353. doi:10.1088/0957-4484/16/10/059
Nicewarner-Peña SR, Freeman RG, Reiss BD, He L, Peña DJ, Walton ID et al (2001) Submicrometer metallic barcodes. Science 294:137–141. doi:10.1126/science.294.5540.137
Pal A, Pal T (1999) Silver nanoparticle aggregate formation by a photochemical method and its application to SERS analysis. J Raman Spectrosc 30(3):199–204. doi:10.1002/(SICI)1097-4555(199903)30:3<199::AID-JRS359>3.0.CO;2-B
Pasquato L, Pengo P, Scrimin P (2004) Functional gold nanoparticles for recognition and catalysis. J Mater Chem 14:3481–3487. doi:10.1039/b410476e
Petit C, Lixon P, Pileni MP (1993) In situ synthesis of silver nanocluster in AOT reverse micelles. J Phys Chem 97(49):12974–12983. doi:10.1021/j100151a054
Ryan D, Rao SN, Rensmo H, Fitzmaurice D, Preece JA, Wenger S et al (2000) Heterosupramolecular chemistry: recognition initiated and inhibited silver nanocrystal aggregation by pseudorotaxane assembly. J Am Chem Soc 122(26):6252–6257. doi:10.1021/ja0002621
Saenger W (1998) Principles of nucleic acid structure. Springer, New York
Sarathy KV, Raina G, Yadav RT, Kulkarni GU, Rao CNR (1997) Thiol-derivatized nanocrystalline arrays of gold, silver, and platinum. J Phys Chem B 101(48):9876–9880. doi:10.1021/jp971544z
Schultz S, Smith DR, Mock JJ, Schultz DA (2000) Single-target molecule detection with nonbleaching multicolor optical immunolabels. Proc Natl Acad Sci USA 97(3):996–1001. doi:10.1073/pnas.97.3.996
Shiraishi Y, Toshima N (1999) Colloidal silver catalysts for oxidation of ethylene. J Mol Catal Chem 141:187–192. doi:10.1016/S1381-1169(98)00262-3
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci 275(1):177–182. doi:10.1016/j.jcis.2004.02.012
Thomson NR (1973) Comprehensive inorganic chemistry. Pergamon Press, New York
Turkevich J, Garton G, Stevenson PC (1954) The color of colloidal gold. J Colloid Sci 9:26–35. doi:10.1016/0095-8522(54)90070-7
Vorobyova SA, Lesnikovich AI, Sobal NS (1999) Preparation of silver nanoparticles by interphase reduction. Colloids Surf A Physicochem Eng Asp 152(3):375–379. doi:10.1016/S0927-7757(98)00861-9
Xu J, Han X, Liu H, Hu Y (2006) Synthesis and optical properties of silver nanoparticles stabilized by gemini surfactant. Colloids Surf A Physicochem Eng Asp 273:179–183. doi:10.1016/j.colsurfa.2005.08.019
Zhang J, Malicka J, Gryczynski I, Lakowicz JR (2004) Oligonucleotide-displaced organic monolayer-protected silver nanoparticles and enhanced luminescence of their salted aggregates. Anal Biochem 330(1):81–86. doi:10.1016/j.ab.2004.04.001
Zheng J, Stevenson MS, Hikida RS, Van Patten PG (2002) Influence of pH on dendrimer-protected nanoparticles. J Phys Chem B 106(6):1252–1255. doi:10.1021/jp013108p
Acknowledgment
The authors would like to thank the National Science Council of Taiwan for their generous financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
11051_2008_9493_MOESM1_ESM.pdf
MOESM1 [Supporting material includes the photographs of 32-MCPAC-Ag-NPs in the presence of phosphate ions over time.] (PDF 55 kb)] (PDF 55 kb)
Rights and permissions
About this article
Cite this article
Misra, T.K., Liu, CY. Surface-functionalization of spherical silver nanoparticles with macrocyclic polyammonium cations and their potential for sensing phosphates. J Nanopart Res 11, 1053–1063 (2009). https://doi.org/10.1007/s11051-008-9493-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-008-9493-x