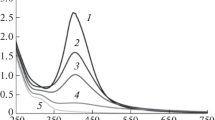
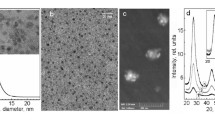
Abstract
Nanoparticles of AgBr were prepared by precipitating AgBr in the water pools of microemulsions consisting of CTAB, n-butanol, isooctane and water. An aqueous solution of AgNO3 added to the microemulsion was the source of Ag+ ions. The formation of AgBr nanoparticles in microemulsions through direct reaction with the surfactant counterion is a novel approach aimed at decreasing the role of intermicellar nucleation on nanoparticle formation for rapid reactions. The availability of the surfactant counterion in every reverse micelle and the rapidity of the reaction with the counterion trigger nucleation within individual reverse micelles. The effect of the following variables on the particle size and size distribution was investigated: the surfactant and cosurfactant concentrations, moles of AgNO3 added, and water to surfactant mole ratio, R. High concentration of the surfactant or cosurfactant, or high water content of the microemulsion favored intermicellar nucleation and resulted in the formation of large particles with broad size distribution, while high amounts of AgNO3 favored nucleation within individual micelles and resulted in small nanoparticles with narrow size distribution. A blue shift in the UV absorption threshold corresponding to a decrease in the particle size was generally observed. Notably, the variation of the absorption peak size with the nanoparticle size was opposite to those reported by us in previous studies using different surfactants.
Similar content being viewed by others

References
Bagwe R.P., Khilar K.C. (1997). Effects of intermicellar exchange rate and cations on the size of silver chloride nanoparticles formed in reverse micelles of AOT. Langmuir 13:6432–6432
Bagwe R.P., Khilar K.C. (2000). Effects of intermicellar exchange rate on the formation of silver nannoparticles in reverse microemulsions of AOT. Langmuir 16:905–910
Belloni J., Mostafavi M., Marignier J., Amblard J. (1991). Quantum size effects and photographic development. J. Imaging Sci. 35:68–74
Bommarius A.S., Holzwarth J.F., Wang D.I., Hatton A.T. (1990). Coalescence and solubilizate exchange in a cationic four-component reversed micellar system. J Phys Chem. 94:7232–7339
Chen W., McLendon G., Marchetti A., Rehm J.M., Freedhoff M.I., Myers C. (1994). Size dependence of radiative rates in the indirect band gap material AgBr. J. Am. Chem. Soc. 116:1585–1586
Chew C.H., Gan L.M., Shah D.O. (1990). The effect of alkanes on the formatin of ultrafine silver bromide particles in ionic w/o microemulsions. J. Dispersion Sci. Technol. 11:593–609
Correa N.M., Zhang H., Schelly Z.A. (2000). Preparation of AgBr quantum dots via electroporation of vesicles. J. Am. Chem. Soc. 122:6432–6434
Curri M.L., Agostiano A., Manna L., Monica M.D., Catalano M., Chiavarone L., Spagnolo V., Lugara M. (2000). Synthesis and characterization of CdS nanoclusters in a quaternary microemulsion: the role of the cosurfactant. J. Phys. Chem. B 104:8391–8397
Fang X., Yang C.J. (1999). An experimental study on the ralationship between the physical properties of CTAB/Hexanol/Water reverse micelles and ZrO2-Y2O3 nanoparticles prepared. Colloid Interface Sci. 212:242–251
Hirai T., Sato H., Komasawa I. (1993). Mechanism of formation of titanium dioxide ultrafine particles in reverse micelles by hydrolysis of titanium tetrabutoxide. Ind Eng Chem Res. 32:3014–3019
Hirai T., Sato H., Komasawa I. (1994). Mechanism of formation of CdS and ZnS ultrafine particles in reverse micelles. Ind. Eng. Chem. Res. 33:3262–3266
Husein M.M., Rodil E., Vera J.H. (2003). Formation of silver chloride nanoparticles in microemulsions by direct precipitaion with the surfactant counterion. Langmuir 19:8467–8474
Husein M.M., Rodil E., Vera J.H. (2004). Formation of silver bromide precipitate of nanoparticles in a single microemulsion utilizing the surfactant counterion. J. Colloid Interface Sci. 273:426–434
Husein M.M., Weber M., Vera J.H. (2001). Nucleophilic substitution sulfonation in emulsions: formation of sodium benzyl sulfonate. Can. J. Chem. Eng. 79:744–750
Johansson K., Marchetti A., McLendon G. (1992). Effect of size restriction on the static and dynamic emission behavior of silver bromide. J. Phys. Chem. 96:2873–2879
Kakuta N., Goto N., Ohkita H., Mizushima T. (1999). Silver bromide as a photocatalyst for hydrogen generatin from CH3OH/H2O solution. J. Phys. Chem. B 103:5917–5919
Monnoyer Ph., Fonseca A., Nagy J. (1995). Preparation of colloidal AgBr particles from microemulsions. Colloids Surf. A 100:233–243
Pillai V., Kumar P., Hou M., Ayyub P., Shah D. (1995). Preparation of nanoparticles of silver halides, Superconductors and magnetic materials using water-in-oil microemulsions as nano-reactors. Adv. Colloid Interface Sci. 55:241–269
Sugimoto T., Shiba F. (2000). Spontaneous nucleation of monodisperse silver halide particles from homogeneous gelatin solution 11: silver bromide. Colloids Surf. A 164:205–215
Tanaka T., Saijo H., Matsubara T. (1979). Optical absorption studies of the growth of microcrystals in nascent silver lodide hydrosols. J. Photogr. Sci. 27:60–65
Towey T.F., Khan-Lodhi A., Robinson B.H. (1990). Kinetics and mechanism of formation of quantum-Sized cadmium sulphide particles in water-aerosol-OT-oil Microemulsions. J. Chem. Soc. Faraday Trans. 86:3757–3762
Vossmeyer T., Katsikas L., Giersig M., Popovic I., Diesner K., Chemseddine A. (1994). Characterization, size dependent oscillatior strength, temperature shift of the excitonic transition energy, and reversible absorbance shift. J. Phys. Chem. 98:7665–7673
Wang W., Weber M.E., Vera J.H. (1994). Effect of alcohol and salt on water uptake of reverse micelles formed by dioctyldimethylammonium chloride (DODMAC) in isooctane. J. Colloid Interface. Sci. 168:422–427
Weller H., Schmidt H.M., Koch U., Fojtik A., Baral S., Henglein A., Kunath W., Weiss K., Dieman E. (1986). Photochemistry of colloidal semiconductors onset of light absorption as a function of size of extremely small CdS particles. Chem. Phys. Lett. 124:557–560
Zhang H., Mostafavi M. (1997). UV-absorption observation of the silver bromide growth from a single molecule to the crystal in solution. J. Phys. Chem. B. 101:8443–8448
Zhang H., Schelly Z.A., Marynick D.S. (2000). Theoretical study of the molecular and electronic structures of neutral silver bromide clusters (AgBr)n, n = 1–9. J. Phys. Chem. A 104:6287–6294
Acknowledgements
The authors thank the Natural Sciences and Engineering Research Council of Canada and the Ministerio de Ciencia y Tecnologia of Spain (project 2004/PC059) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Husein, M.M., Rodil, E. & Vera, J.H. Preparation of AgBr Nanoparticles in Microemulsions Via Reaction of AgNO3 with CTAB Counterion. J Nanopart Res 9, 787–796 (2007). https://doi.org/10.1007/s11051-006-9107-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-006-9107-4