Abstract
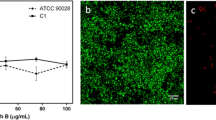
Candida albicans persisters have so far been observed only in biofilm environment; the biofilm element(s) that trigger(s) persister formation are still unknown. In this study, we tried to further elucidate the possible relationship between C. albicans persisters and the early phases of biofilm formation, especially the surface adhesion phase. Three C. albicans strains were surveyed for the formation of persisters. We tested C. albicans persister formation dynamically at different time points during the process of adhesion and biofilm formation. The number of persister cells was determined based on an assessment of cell viability after amphotericin B treatment and colony-forming unit assay. None of the planktonic cultures contained persisters. Immediately following adhesion of C. albicans cells to the surface, persister cells emerged and the proportion of persisters reached a peak of 0.2–0.69 % in approximately 2-h biofilm. As the biofilm matured, the proportion of persisters decreased and was only 0.01–0.02 % by 24 h, while the number of persisters remained stable with no significant change. Persisters were not detected in the absence of an attachment surface which was pre-coated. Persisters were also absent in biofilms that were scraped to disrupt surface adhesion prior to amphotericin B treatment. These results indicate that C. albicans antifungal-tolerant persisters are produced mainly in surface adhesion phase and surface adhesion is required for the emergence and maintenance of C. albicans persisters.




Similar content being viewed by others
References
Selmecki A, Forche A, Berman J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science. 2006;313(5785):367–70. doi:10.1126/science.1128242.
Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11(1):30–6.
Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch Intern Med. 1988;148(12):2642–5.
Seneviratne CJ, Jin L, Samaranayake LP. Biofilm lifestyle of Candida: a mini review. Oral Dis. 2008;14(7):582–90. doi:10.1111/j.1601-0825.2007.01424.x.
Colombo AL, Nucci M, Park BJ, Nouer SA, Arthington-Skaggs B, da Matta DA, et al. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. J Clin Microbiol. 2006;44(8):2816–23. doi:10.1128/JCM.00773-06.
Wenzel RP, Gennings C. Bloodstream infections due to Candida species in the intensive care unit: identifying especially high-risk patients to determine prevention strategies. Clin Infect Dis. 2005;41(Suppl 6):S389–93. doi:10.1086/430923.
Mathe L, Van Dijck P. Recent insights into Candida albicans biofilm resistance mechanisms. Curr Genet. 2013;59(4):251–64. doi:10.1007/s00294-013-0400-3.
Bouza E, Burillo A, Munoz P, Guinea J, Marin M, Rodriguez-Creixems M. Mixed bloodstream infections involving bacteria and Candida spp. J Antimicrob Chemother. 2013;68(8):1881–8. doi:10.1093/jac/dkt099.
Peng S, Lu Y. Clinical epidemiology of central venous catheter-related bloodstream infections in an intensive care unit in China. J Crit Care. 2013;28(3):277–83. doi:10.1016/j.jcrc.2012.09.007.
Taff HT, Mitchell KF, Edward JA, Andes DR. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013;8(10):1325–37. doi:10.2217/fmb.13.101.
Qu Y, Daley AJ, Istivan TS, Rouch DA, Deighton MA. Densely adherent growth mode, rather than extracellular polymer substance matrix build-up ability, contributes to high resistance of Staphylococcus epidermidis biofilms to antibiotics. J Antimicrob Chemother. 2010;65(7):1405–11. doi:10.1093/jac/dkq119.
Baillie GS, Douglas LJ. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob Agents Chemother. 1998;42(8):1900–5.
Baillie GS, Douglas LJ. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Chemother. 2000;46(3):397–403.
de Micheli M, Bille J, Schueller C, Sanglard D. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol Microbiol. 2002;43(5):1197–214.
Ramage G, Bachmann S, Patterson TF, Wickes BL, Lopez-Ribot JL. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother. 2002;49(6):973–80.
LaFleur MD, Kumamoto CA, Lewis K. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob Agents Chemother. 2006;50(11):3839–46. doi:10.1128/aac.00684-06.
Al-Dhaheri RS, Douglas LJ. Absence of amphotericin B-tolerant persister cells in biofilms of some Candida species. Antimicrob Agents Chemother. 2008;52(5):1884–7. doi:10.1128/aac.01473-07.
LaFleur MD, Qi Q, Lewis K. Patients with long-term oral carriage harbor high-persister mutants of Candida albicans. Antimicrob Agents Chemother. 2009;54(1):39–44. doi:10.1128/aac.00860-09.
Al-Dhaheri RS, Douglas LJ. Apoptosis in Candida biofilms exposed to amphotericin B. J Med Microbiol. 2010;59(Pt 2):149–57. doi:10.1099/jmm.0.015784-0.
Bink A, Vandenbosch D, Coenye T, Nelis H, Cammue BP, Thevissen K. Superoxide dismutases are involved in Candida albicans biofilm persistence against miconazole. Antimicrob Agents Chemother. 2011;55(9):4033–7. doi:10.1128/AAC.00280-11.
Dawson CC, Intapa C, Jabra-Rizk MA. “Persisters”: survival at the cellular level. PLoS Pathog. 2011;7(7):e1002121. doi:10.1371/journal.ppat.1002121.
Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–31.
Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5(1):48–56. doi:10.1038/nrmicro1557.
Lewis K. Persister cells. Annu Rev Microbiol. 2010;64(1):357–72. doi:10.1146/annurev.micro.112408.134306.
Lewis K. Persister cells: molecular mechanisms related to antibiotic tolerance. Handb Exp Pharmacol. 2012;211:121–33. doi:10.1007/978-3-642-28951-4_8.
Spoering AL, Lewis K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J Bacteriol. 2001;183(23):6746–51. doi:10.1128/JB.183.23.6746-6751.2001.
Keren I, Minami S, Rubin E, Lewis K. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. MBio. 2011;2(3):e00100–11. doi:10.1128/mBio.00100-11.
Kwan BW, Valenta JA, Benedik MJ, Wood TK. Arrested protein synthesis increases persister-like cell formation. Antimicrob Agents Chemother. 2013;57(3):1468–73. doi:10.1128/AAC.02135-12.
Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198(1):179–82.
Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90(5):939–49.
Yang C, Gong W, Lu J, Zhu X, Qi Q. Antifungal drug susceptibility of oral Candida albicans isolates may be associated with apoptotic responses to Amphotericin B. J Oral Pathol Med. 2010;39(2):182–7. doi:10.1111/j.1600-0714.2009.00811.x.
Silici S, Koc AN. Comparative study of in vitro methods to analyse the antifungal activity of propolis against yeasts isolated from patients with superficial mycoses. Lett Appl Microbiol. 2006;43(3):318–24. doi:10.1111/j.1472-765X.2006.01949.x.
Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45(9):2475–9.
Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. Biofilm formation by Candida dubliniensis. J Clin Microbiol. 2001;39(9):3234–40.
Mulcahy LR, Burns JL, Lory S, Lewis K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J Bacteriol. 2010;192(23):6191–9. doi:10.1128/JB.01651-09.
Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183(18):5385–94. doi:10.1128/jb.183.18.5385-5394.2001.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant Nos 30973310, 81371158). Our laser confocal scanning work was performed at The Microscopy Characterization Facility, Shandong University. We are also indebted to Professor K. Lewis for supplying the C. albicans strains.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, J., Li, Z., Chu, H. et al. Candida albicans Amphotericin B-Tolerant Persister Formation is Closely Related to Surface Adhesion. Mycopathologia 181, 41–49 (2016). https://doi.org/10.1007/s11046-015-9894-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-015-9894-1