Abstract
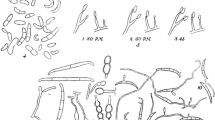
Polymorphic sequence-characterised marker assays from a recent diversity study on the Ascomycete fungus Ophiostoma quercus reported that some isolates from Africa were genetically distinct from O. quercus. In the present study, these African isolates were compared with authentic O. quercus isolates by evaluating morphological characters, growth in culture, mating compatibility and DNA sequence data. The isolates from Africa were morphologically similar to O. quercus, presenting Pesotum and Sporothrix synanamorphs in culture. Phylogenetic analyses of the ribosomal internal transcribed spacer regions 1 and 2, β-tubulin and translation elongation factor 1-α gene regions confirmed that the African group represents a distinct species within the hardwood lineage of the O. piceae complex, closely related to O. ulmi and O. himal-ulmi. Mating studies between O. quercus and the African isolates showed that isolates mated predominantly with those of their own group, although there were rare cases of fertile crosses between the groups. Isolates residing in the African lineage are described here as a new species, O. tsotsi sp. nov.




Similar content being viewed by others
References
Piel F, Gilbert M, De Cannière C, Grégoire J-C. Coniferous round wood imports from Russia and Baltic countries to Belgium. A pathway analysis for assessing risks of exotic pest insect introductions. Divers Distrib. 2008;14:318–28.
Zipfel RD, De Beer ZW, Jacobs K, Wingfield BD, Wingfield MJ. Multi-gene phylogenies define Ceratocystiopsis and Grosmannia distinct from Ophiostoma. Stud Mycol. 2006;55:75–97.
Seifert KA, Wingfield MJ, Kendrick WB. Sapstain of commercial lumber by species of Ophiostoma and Ceratocystis. In: Wingfield MJ, Seifert KA, Webber J, editors. Ceratocystis and Ophiostoma: taxonomy, ecology and pathogenicity. St. Paul, Minnesota: APS Press; 1993. pp. 141–51.
Brasier CM. China and the origins of Dutch elm disease: an appraisal. Plant Pathol. 1990;39:5–16.
Brasier CM. Ophiostoma novo-ulmi sp. nov., causative agent of current Dutch elm disease pandemics. Mycopathol. 1991;115:151–61.
Lin TC, Huang JW, Hsieh WH. Identification of ophiostomatoid fungi associated with Chinese fir wilt in Taiwan [in Chinese]. Plant Pathol Bull. 2003;12:33–42.
Zhou XD, De Beer ZW, Ahumada R, Wingfield BD, Wingfield MJ. Ophiostoma and Ceratocystiopsis spp. associated with two pine-infesting bark beetles in Chile. Fungal Divers. 2004;15:261–74.
Zhou X, De Beer ZW, Wingfield MJ. DNA sequence comparisons of Ophiostoma spp., including Ophiostoma aurorae sp. nov., associated with pine bark beetles in South Africa. Stud Mycol. 2006;55:269–77.
Carlier F-X, Decock C, Jacobs K, Maraite H. Ophiostoma arduennense sp. nov. (Ophiostomatales, Ascomycota) from Fagus sylvatica in southern Belgium. Mycol Res. 2006;110:801–10.
Romón P, Zhou X, Iturrondobeitia JC, Wingfield MJ, Goldarazena A. Ophiostoma species (Ascomycetes: Ophiostomatales) associated with bark beetles (Coleoptera: Scolytinae) colonizing Pinus radiata in northern Spain. Can J Microbiol. 2007;53:756–67.
Linnakoski R, De Beer ZW, Rousi M, Niemelä P, Pappinen A, Wingfeld MJ. Fungi including Ophiostoma karelicum sp. nov., associated with Scolytus ratzeburgi infesting birch in Finland and Russia. Mycol Res. 2008;112:1475–88.
Geldenhuis MM, Roux J, Montenegro F, De Beer ZW, Wingfield MJ, Wingfield BD. Identification and pathogenicity of Graphium and Pesotum species from machete wounds on Schizolobium parahybum in Ecuador. Fungal Divers. 2004;15:135–49.
Kamgan Nkuekam G, Jacobs K, De Beer ZW, Wingfield MJ, Roux J. Ceratocystis and Ophiostoma species including three new taxa, associated with wounds on native South African trees. Fungal Divers. 2008;29:37–59.
Kamgan Nkuekam G, Jacobs K, De Beer ZW, Wingfield MJ, Roux J. Pesotum australi sp. nov. and Ophiostoma quercus associated with Acacia mearnsii trees in Australia and Uganda, respectively. Australas Plant Pathol. 2008;37:406–16.
Grobbelaar J, Aghayeva D, De Beer ZW, Bloomer P, Wingfield M, Wingfield B. Delimitation of Ophiostoma quercus and its synonyms using multiple gene phylogenies. Mycol Prog. 2009;8:221–36.
De Beer ZW, Wingfield BD, Wingfield MJ. The occurrence of Ophiostoma piliferum-like fungi on pulpwood chips and other wood sources in South Africa. S A J Sci. 2003;99:34–6.
De Beer ZW, Wingfield BD, Wingfield MJ. The Ophiostoma piceae complex in the southern hemisphere: a phylogenetic study. Mycol Res. 2003;107:469–76.
Kim G-H, Kim J-J, Lim YW, Breuil C. Ophiostomatoid fungi isolated from Pinus radiata logs imported from New Zealand to Korea. Can J Bot. 2005;83:272–8.
Kim G-H, Kim J-J, Breuil C. Sap-staining fungi from logs and boards of two commercially important pines in Korea. Holzforschung. 2007;61:333–6.
Thwaites JM, Farrell RL, Hata K, Carter P, Lausberg M. Sapstain fungi on Pinus radiata logs—from New Zealand forest to export in Japan. J Wood Sci. 2004;50:459–65.
Thwaites JM, Farrell RL, Duncan SM, Reay SD, Blanchette RA, Hadar E, et al. Survey of potential sapstain fungi on Pinus radiata in New Zealand. N Z J Bot. 2005;43:653–63.
Grobbelaar JW, Barnes I, Cortinas M-N, Bloomer P, Wingfeld MJ, Wingfeld BD. Development and characterization of polymorphic markers for the sap-stain fungus Ophiostoma quercus. Mol Ecol Resour. 2008;9:399–401.
Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes––application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–8.
White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and application. San Diego: Academic Press; 1990. p. 315–22.
O’Donnell K, Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol. 1997;7:103–16.
Glass NL, Donaldson GC. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Appl Environ Microbiol. 1995;61:1323–30.
Jacobs K, Bergdahl DR, Wingfield MJ, Halik S, Seifert KA, Bright DE, et al. Leptographium wingfieldii introduced into North America and found associated with exotic Tomicus piniperda and native bark beetles. Mycol Res. 2004;108:411–8.
Katoh K, Toh H. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform. 2008;9:286–98.
Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evol. 1985;39:783–91.
Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–8.
Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–4.
Upadhyay HP. A monograph of Ceratocystis and Ceratocystiopsis. Athens: University of Georgia Press; 1981.
Kowalski T, Butin H. Taxonomie bekannter und neuer Ceratocystis-Arten an Eiche (Quercus robur L). J Phytopathol. 1989;124:236–48.
Bickford D, Lohman DJ, Sodhi NS, Ng PKL, Meier R, Winker K, et al. Cryptic species as a window on diversity and conservation. Trends Ecol Evol. 2007;22:148–55.
Noor MAF, Feder JL. Speciation genetics: evolving approaches. Nat Rev Gen. 2006;7:851–61.
Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, et al. Phylogenetic species recognition and species concepts in fungi. Fungal Genet Biol. 2000;31:21–32.
Engelbrecht CJB, Harrington TC. Intersterility, morphology and taxonomy of Ceratocystis fimbriata on sweet potato, cacao and sycamore. Mycologia. 2005;97:57–69.
Kohn LM. Mechanisms of fungal speciation. Ann Rev Phytopathol. 2005;43:279–308.
Brasier CM. The genetic system as a fungal taxonomic tool: gene flow, molecular variation and sibling species in the ‘Ophiostoma piceae––O. ulmi’ complex and its taxonomic and ecological significance. In: Wingfield MJ, Seifert KA, Webber J, editors. Ceratocystis and Ophiostoma: taxonomy, ecology and pathogenicity. St. Paul, Minnesota: APS Press; 1993. pp. 77–92.
Harrington TC, McNew DL. Partial interfertility among the Ceratocystis species on conifers. Fungal Genet Biol. 1998;25:44–53.
Harrington TC, McNew D, Steimel J, Hofstra D, Farrell R. Phylogeny and taxonomy of the Ophiostoma piceae complex and the Dutch Elm disease fungi. Mycologia. 2001;93:111–36.
Acknowledgments
We thank Jolanda Roux, Ronald Heath and other colleagues who assisted with the collection of isolates. We acknowledge our African collaborators: Gerald Meke from the Forestry Research Institute of Malawi, Aza Mbaga from the Tanganyika Wattle Company, Tanzania, the Forestry Department and Makerere University, Uganda. We are also grateful to Dr. Seonju Marincowitz for assisting with light microscopy, Mr Alan Hall for assistance with scanning electron microscopy and Dr. Hugh Glen for providing the Latin diagnosis. Funding for this study was provided by the National Research Foundation (NRF), the Tree Protection Co-operative Programme (TPCP), the THRIP initiative of the Department of Trade and Industry (DTI) and the Department of Science and Technology (DST)/NRF Centre of Excellence in Tree Health Biotechnology, South Africa.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grobbelaar, J.W., de Beer, Z.W., Bloomer, P. et al. Ophiostoma tsotsi sp. nov., A Wound-infesting Fungus of Hardwood Trees in Africa. Mycopathologia 169, 413–423 (2010). https://doi.org/10.1007/s11046-009-9267-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-009-9267-8