Abstract
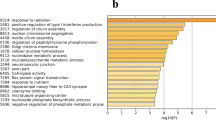
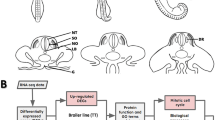
Transition from laying to incubation behavior in chicken is an interesting topic in reproductive biology. The decline of incubation behavior in chicken population has led to considerable phenotypic differences in reproductive traits between breeds. However, the exact genetic mechanism of the reproductive phase transition still largely unknown and little is known about the gene expression changes that contribute to the phenotypic differences. We performed mRNA sequencing to investigate the molecular mechanism underlying the transition from laying to brooding and to detect difference in gene regulation underlying the phenotypic diversification using two chicken breeds. The majority of gene expression changes during phase transition were steroidogenesis and hormone-releasing genes. Brooding chickens shared a conservative pattern of greatly inhibited steroidogenic enzyme genes in the pituitary gland, therefore, low levels of steroidogenic enzymes might result in reproductive defects such as ovary regression and brooding onset. The conserved network responsible for brooding behavior was maintained by steroid biosynthesis and hormonal interactions. Interestingly, three transcription factors, SREBF2, NR5A1 and PGR, act as central signal modulators of steroid biosynthesis and hormonal interactions during the transition from laying to brooding modes at the molecular level. Furthermore, Genes correlated with protein synthesis and accumulation showed expression variation between breeds, which might result in different concentrations of and sensitivities to reproduction-related hormones. This study provided a new insight in neuroendocrine system at the molecular level, and helps to understand the genetic and hormonal responses that ultimately translate into behavior in chicken.



Similar content being viewed by others
References
Youngren O, Chaiseha Y, Phillips R, El Halawani M (1996) Vasoactive intestinal peptide concentrations in turkey hypophysial portal blood differ across the reproductive cycle. Gen Comp Endocrinol 103(3):323–330
Ramesh R, Kuenzel WJ, Proudman JA (2001) Increased proliferative activity and programmed cellular death in the turkey hen pituitary gland following interruption of incubation behavior. Biol Reprod 64(2):611–618
Zhou M, Du Y, Nie Q, Liang Y, Luo C, Zeng H et al (2010) Associations between polymorphisms in the chicken VIP gene, egg production and broody traits. Br Poult Sci 51(2):195–203
Romanov MN, Talbot RT, Wilson PW, Sharp PJ (2002) Genetic control of incubation behavior in the domestic hen. Poult Sci 81(7):928–931
Dunn IC, Chen Y, Hook C, Sharp PJ, Sang HM (1993) Characterization of the chicken preprogonadotrophin -releasing hormone-I gene. J Mol Endocrinol 11(1):19–29
Dunn IC, McEwan G, Okhubo T, Sharp PJ, Paton IR et al (1998) Genetic mapping of the chicken prolactin receptor gene: a candidate gene for the control of broodiness. Br Poult Sci 39(Suppl):S23–S24
Basheer A, Wilson PW, Talbot RJ, Sharp PJ, Law A, et al. (2010) dissecting the genetics of maternal behavior in chickens [abstract]. 32nd conference of the International Society for Animal Genetics
Dunn IC, Miao YW, Morris A, Romanov MN, Wilson PW et al (2004) A study of association between genetic markers in candidate genes and reproductive traits in one generation of a commercial broiler breeder hen population. Heredity 92(2):128–134
Cui JX, Du HL, Liang Y, Deng XM, Li N et al (2006) Association of polymorphisms in the promoter region of chicken prolactin with egg production. Poult Sci 85(1):26–31
Jiang RS, Xu GY, Zhang XQ, Yang N (2005) Association of polymorphisms for prolactin and prolactin receptor genes with broody traits in chickens. Poult Sci 84(6):839–845
Liang Y, Cui J, Yang G, Leung FC, Zhang X (2006) Polymorphisms of 5′ flanking region of chicken prolactin gene. Domest Anim Endocrinol 30(1):1–16
Zhou M, Lei M, Rao Y, Nie Q, Zeng H et al (2008) Polymorphisms of vasoactive intestinal peptide receptor-1 gene and their genetic effects on broodiness in chickens. Poult Sci 87(5):893–903
Xu H, Shen X, Zhou M, Fang M, Zeng H et al (2010) The genetic effects of the dopamine D1 receptor gene on chicken egg production and broodiness traits. BMC Genet 11:17
Sharp PJ (2004) Genes for persistency of egg laying: White Leghorns and broodiness. Rosl Inst Edinb Annl Rep xx(ss):38–42
EL Halawani ME, Silsby JL, Behnke EJ, Fehrer SC (1986) Hormonal induction of incubation behavior in ovariectomized female turkeys (Meleagris gallopavo). Biol Reprod 35(1):59–67
Youngren OM, el Halawani ME, Silsby JL, Phillips RE (1991) Intracranial prolactin perfusion induces incubation behavior in turkey hens. Biol Reprod 44(3):425–431
Sharp PJ, Sterling RJ, Talbot RT, Huskisson NS (1989) The role of hypothalamic vasoactive intestinal polypeptide in the maintenance of prolactin secretion in incubating bantam hens: observations using passive immunization, radioimmunoassay and immunohistochemistry. J Endocrinol 122(1):5–13
Buntin JD, Lea RW, Figge GR (1988) Reductions in plasma LH concentration and testicular weight in ring doves following intracranial injection of prolactin or growth hormone. J Endocrinol 118(1):33–40
Halawani EIME, Burke WH, Dennison PT (1980) Effect of nest-deprivation on serum prolactin level in nesting female turkeys. Biol Reprod 23(1):118–123
Bhatt R, Youngren O, Kang S, El Halawani M (2003) Dopamine infusion into the third ventricle increases gene expression of hypothalamic vasoactive intestinal peptide and pituitary prolactin and luteinizing hormone beta subunit in the turkey. Gen Comp Endocrinol 130(1):41–47
Chaiseha Y, Youngren O, Al-Zailaie K, El Halawani ME (2003) Expression of D1 and D2 dopamine receptors in the hypothalamus and pituitary during the turkey reproductive cycle: colocalization with vasoactive intestinal peptide. Neuroendocrinology 77(2):105–118
El Halawani ME, Silsby JL, Youngren OM, Phillips RE (1991) Exogenous prolactin delays photo-induced sexual maturity and suppresses ovariectomy-induced luteinizing hormone secretion in the turkey (Meleagris gallopavo). Biol Reprod 44(3):420–424
Kulick RS, Chaiseha Y, Kang SW, Rozenboim I, El Halawani ME (2005) The relative importance of vasoactive intestinal peptide and peptide histidine isoleucine as physiological regulators of prolactin in the domestic turkey. Gen Comp Endocrinol 142(3):267–273
Porter TE, Lopez ME, Mike R, Huberty AF (2006) The increase in prolactin-secreting cells in incubating chicken hens can be mimicked by extended treatment of pituitary cells in vitro with vasoactive intestinal polypeptide (VIP). Domest Anim Endocrinol 30(2):126–134
Thayananuphat A, Youngren OM, Kang SW, Bakken T, Kosonsiriluk S (2011) Dopamine and mesotocin neurotransmission during the transition from incubation to brooding in the turkey. Horm Behav 60(4):327–335
Prakobsaeng N, Sartsoongnoen N, Kosonsiriluk S, Chaiyachet OA, Chokchaloemwong D et al (2011) Changes in vasoactive intestinal peptide and tyrosine hydroxylase immunoreactivity in the brain of nest-deprived native Thai hen. Gen Comp Endocrinol 171:189–196
Ramesh R, Proudman JA, Kuenzel WJ (1996) Changes in pituitary somatotroph and lactotroph distribution in laying and incubating turkey hens. Gen Comp Endocrinol 104(1):67–75
Rockman MV, Kruglyak L (2006) Genetics of global gene expression. Nat Rev Genet 7(11):862–872
Albert FW, Somel M, Carneiro M, Aximu-Petri A, Halbwax M et al (2012) A comparison of brain gene expression levels in domesticated and wild animals. PLoS Genet 8(9):e1002962
Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26(5):589–595
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5(7):621–628
Wang L, Feng Z, Wang X, Wang X, Zhang X (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26(1):136–138
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R stat soc 57(1):289–300
R development Core Team: R (2008) A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
Gaccioli F, White V, Capobianco E, Powell TL, Jawerbaum A et al (2013) Maternal overweight induced by a diet with high content of saturated fat activates placental mTOR and eIF2alpha signaling and increases fetal growth in rats. Biol Reprod 89(4):96
Hunter MG, Robinson RS, Mann GE, Webb R (2004) Endocrine and paracrine control of follicular development and ovulation rate in farm species. Anim Reprod Sci 82–83:461–477
Nivet AL, Vigneault C, Blondin P, Sirard MA (2013) Changes in granulosa cells’ gene expression associated with increased oocyte competence in bovine. Reproduction 145(6):555–565
Hansen C, Yi N, Zhang YM, Xu S, Gavora J et al (2005) Identification of QTL for production traits in chickens. Anim Biotechnol 16(1):67–79
Macnamee MC, Sharp PJ (1989) The functional activity of hypothalamic 5-hydroxytryptamine neurones in broody bantam hens. J Endocrinol 120(1):125–134
Porter TE, Silsby JL, Behnke EJ, Knapp TR, Halawani ME (2001) Ovarian steroid production in vitro during gonadal regression in the turkey. I. changes associated with incubation behavior. Biol Reprod 45(4):581–586
Hanukoglu I (1992) Steroidogenic enzymes: structure, function, and role in regulation of steroid hormone biosynthesis. J Steroid Biochem Mol Biol 43(8):779–804
Goldsmith AR (1991) Prolactin and avian reproductive strategies. Acta XX Congressus Inter Ornithologici 4:2063–2071
Vleck CM (2002) Hormonal control of incubation behaviour. In: Deeming DC (ed) Avian incubation: behaviour, environment and evolution. Oxford University Press, New York, pp 54–62
Nett TM, Turzillo AM, Baratta M, Rispoli LA (2002) Pituitary effects of steroid hormones on secretion of follicle-stimulating hormone and luteinizing hormone. Domest Anim Endocrinol 23(1–2):33–42
Dias FC, Khan MI, Sirard MA, Adams GP (2013) Singh J (2013) differential gene expression of granulosa cells after ovarian superstimulation in beef cattle. Reproduction 146(2):181–191
Guémené D, Williams JB (1994) Relationships between broodiness expression laying persistency and concentrations of hormones during the first productive period in turkey hens (Meleagris gallopavo). Reprod Nutr Dev 34(4):371–381
Porter TE, Hargis BM, Silsby JL, el Halawani ME (1989) Enhanced progesterone and testosterone secretion and depressed estradiol secretion in vitro from small white follicle cells of incubating turkey hens. Gen Comp Endocrinol 74(3):400–405
Higgins SE, Ellestad LE, Trakooljul N, McCarthy F, Saliba J (2010) Transcriptional and pathway analysis in the hypothalamus of newly hatched chicks during fasting and delayed feeding. BMC Genom 11:162
McGowan BM, Stanley SA, Donovan J, Thompson EL, Patterson M (2008) Relaxin-3 stimulates the hypothalamic-pituitary-gonadal axis. Am J Physiol Endocrinol Metab 295(2):E278–E286
Kallingal GJ, Mintz EM (2007) Gastrin releasing peptide and neuropeptide Y exert opposing actions on circadian phase. Neurosci Lett 422(1):59–63
Liu Z, Rudd MD, Hernandez-Gonzalez I, Gonzalez-Robayna I, Fan HY (2009) FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Mol Endocrinol 23(5):649–661
Mallet D, Bretones P, Michel-Calemard L, Dijoud F, David M et al (2004) Gonadal dysgenesis without adrenal insufficiency in a 46, XY patient heterozygous for the nonsense C16X mutation: a case of SF1 haploinsufficiency. Clin Endocrinol Metab 89(10):4829–4832
Hasegawa T, Fukami M, Sato N, Katsumata N, Sasaki G et al (2004) Testicular dysgenesis without adrenal insufficiency in a 46, XY patient with a heterozygous inactive mutation of steroidogenic factor-1. J Clin Endocrinol Metab 89(12):5930–5935
Jameson JL (2004) Of mice and men: the tale of steroidogenic factor-1. J Clin Endocrinol Metab 89(12):5927–5929
Luo X, Ikeda Y, Parker KL (1994) A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77(4):481–490
Sockman KW, Schwabl H (1999) Daily estradiol and progesterone levels relative to laying and onset of incubation in canaries. Gen Comp Endocrinol 114(2):257–268
Rubin CJ, Zody MC, Eriksson J, Meadows JR, Sherwood E et al (2010) Whole-genome resequencing reveals loci under selection during chicken domestication. Nature 464(7288):587–591
Devost D, Carrier ME, Zingg HH (2008) Oxytocin-induced activation of eukaryotic elongation factor 2 in myometrial cells is mediated by protein kinase C. Endocrinology 149(1):131–138
Burgos SA, Dai M, Cant JP (2010) Nutrient availability and lactogenic hormones regulate mammary protein synthesis through the mammalian target of rapamycin signaling pathway. J Dairy Sci 93(1):153–161
Bulfield G, Isaacson JH, O’Mara H (1988) Sensitivity of the oviduct to oestrogens in broiler and layer chickens: differential response in the induction of ovalbumin gene expression. Theor Appl Genet 75:783–799
Hocking PM, McCormack HA (1995) Differential sensitivity of ovarian follicles to gonadotrophin stimulation in broiler and layer lines of domestic fowl. J Reprod Fertil 105(1):49–55
Liu HK, Long DW, Bacon WL (2002) Interval between preovulatory surges of luteinizing hormone increases late in the reproductive period in turkey hens. Biol Reprod 66(4):1068–1075
Reddy IJ, David CG, Raju SS (2007) Effect of suppression of plasma prolactin on luteinizing hormone concentration, intersequence pause days and egg production in domestic hen. Domest Anim Endocrinol 33(2):167–175
Hocking PM, Gilbert AB, Walker M, Waddington D (1987) Ovarian follicular structure of White Leghorns fed ad libitum and dwarf and normal broiler breeders fed ad libitum or restricted until point of lay. Br Poult Sci 28(3):493–506
Nili H, Kelly WR (1996) Form and function of lacunae in the ovary of the laying hen. Anat Rec 244(2):165–174
Acknowledgments
We thank Weiwei Zhai, Jue Ruan, Yu Wang, Gong Qiang and Juan Li of the Beijing Institute of Genomics, Chinese Academy of Science, for important comments on the data analysis. We thank the Guangfeng Baier Huang Breeding Farm, Shangrao city, Jiangxi province, China, for providing BEH chickens. We also thank Liyan Jiang, Hua Zeng and Weimin Li for collecting the XH chicken tissues and Dr. Hongli Du for beneficial discussions and useful advice.
Funding
This work was supported by the following grants: China Agriculture Research System (CARS-42-G05), China High-Tech Programs (2013AA102501), National Natural Science Foundation of China (31301010), as well as the China Postdoctoral Science Foundation (2012M520359).
Author contributions
Xu Shen, Xuemei Lu, Qinghua Nie and Xiquan Zhang conceived and designed the experiments. Xu Shen performed the experiments. Xue Bai, Jin Xu and Xu Shen contributed to data analysis. Min Zhou and Haipin Xu helped to collect the chicken samples. Xu Shen and Xiquan Zhang wrote the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Ethics statement
This study was approved by the Animal Care Committee of South China Agricultural University (Guangzhou, China) under approval number SCAU#0016. The animals used in this study were humanely sacrificed as needed to ameliorate their suffering.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shen, X., Bai, X., Xu, J. et al. Transcriptome sequencing reveals genetic mechanisms underlying the transition between the laying and brooding phases and gene expression changes associated with divergent reproductive phenotypes in chickens. Mol Biol Rep 43, 977–989 (2016). https://doi.org/10.1007/s11033-016-4033-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-016-4033-8