Abstract
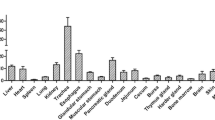
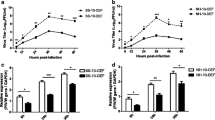
Cluster of differentiation 8 alpha (CD8α) is critical for cell-mediated immune defense and T-cell development. Although CD8α sequences have been reported for several species, very little is known about CD8α in ducks. To elucidate the mechanisms involved in the innate and adaptive immune responses of ducks, we cloned CD8α coding sequences from domestic, Muscovy, Mallard, and Spotbill ducks using reverse transcription polymerase chain reaction (RT-PCR). Each sequence consisted of 714 nucleotides and encoded a signal peptide, an IgV-like domain, a stalk region, a transmembrane region, and a cytoplasmic tail. We identified 58 nucleotide differences and 37 amino acid differences among the four types of duck; of these, 53 nucleotide and 33 amino acid differences were between Muscovy ducks and the other duck species. The CD8α cDNA sequence from domestic duck consisted of a 61-nucleotide 5′ untranslated region (UTR), a 714-nucleotide open reading frame, and an 849-nucleotide 3′ UTR. Multiple sequence alignments showed that the amino acid sequence of CD8α is conserved in vertebrates. RT-PCR revealed that expression of CD8α mRNA of domestic ducks was highest in the thymus and very low in the kidney, cerebrum, cerebellum, and muscle. Immunohistochemical analyses detected CD8α on the splenic corpuscle and periarterial lymphatic sheath of the spleen. CD8α mRNA in domestic ducklings was initially up-regulated, and then down-regulated, in the thymus, spleen, and liver after treatment with duck hepatitis virus type I (DHV-1) or the immunostimulant polyriboinosinic polyribocytidylic acid (poly I:C).





Similar content being viewed by others
References
Huang YH, Li N, Burt DW, Wu F (2008) Genomic research and applications in the duck (Anas platyrhynchos). World Poult Sci J 64:329–341
van Loenen MM, Hagedoorn RS, de Boer R, Falkenburg JH, Heemskerk MH (2013) Extracellular domains of CD8α and CD8ß subunits are sufficient for HLA class I restricted helper functions of TCR-engineered CD4(+) T cells. PLoS ONE 30:e65212
Abbas AK, Lichtman AH (2003) Cellular and molecular immunology, 5th edn. Saunders, Philadelphia
Hu Q, Pan Z, Deen S, Meng S, Zhang X, Zhang X, Jiao XA (2007) New alleles of chicken CD8α and CD3ε found in Chinese native and western breeds. Vet Immunol Immunopathol 120:223–233
Tang XS, Wang L, Liao DW, Jiang YN, Xia C (2007) Cloning, expression and production of polyclonal antibodies in extracellular regions of chicken CD8α/β. J China Agr Univ 12:5–9
Liaw HJ, Chen WR, Huang YC, Tsai CW, Chang KC, Kuo CL (2007) Genomic organization of the chicken CD8 locus reveals a novel family of immunoreceptor genes. J Immuno 178:3023–3030
Tregaskes CA, Kong FK, Paramithiotis E, Chen CL, Ratcliffe MJ, Davison TF, Young JR (1995) Identification and analysis of the expression of CD8αβ and CD8αα isoforms in chickens reveals a major TCR-γδ CD8αβ subset of intestinal intraepithelial lymphocytes. J Immunol 154:4485–4494
Kothlow S, Mannes NK, Schaerer B, Rebeski DE, Kaspers B, Schultz U (2005) Characterization of duck leucocytes by monoclonal antibodies. Dev Comp Immunol 29:733–748
Fletcher OJ, Tan X, Cortes L (2012) Cost effective and time efficient measurement of CD4, CD8, major histocompatibility complex class II, and macrophage antigen expression in the lungs of chickens. Vet Immunol Immunopathol 146:225–236
Reaiche GY (2008) Characterization of the events involved in the resolution of acute duck hepatitis B virus infection. Thesis of the University of Adelaide, Adelaide
Mancebo E, Moreno-Pelayo MA, Mencía A, de la Calle-Martín O, Allende LM, Sivadorai P, Kalaydjieva L, Bertranpetit J, Coto E, Calleja-Antolín S, Ruiz-Contreras J, Paz-Artal E (2008) Gly111Ser mutation in CD8A gene causing CD8 immunodeficiency is found in Spanish gypsies. Mol Immunol 45:479–484
de la Calle-Martin O, Hernandez M, Ordi J, Casamitjana N, Arostegui JI, Caragol I, Ferrando M, Labrador M, Rodriguez-Sanchez JL, Espanol T (2001) Familial CD8 deficiency due to a mutation in the CD8 alpha gene. J Clin Invest 108:117–123
Jin X, Zhang W, Zhang W, Gu C, Cheng G, Hu X (2008) Identification and molecular analysis of the highly pathogenic duck hepatitis virus type I in Hubei province of China. Res Vet Sci 85:595–598
Cheng HJ, Cheng AC, Wang MS, Chen XY (2007) Development of an indirect immunoperoxidase staining technique for the detection of duck hepatitis virus type 1. Vet Sci China 37:369–373
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Hu XY, Cheng GF, Zhou SQ, Xiong DH (2000) Studies on histopathological changes of inoculated ducklings with DHV-1. J Huazhong Agric Univ 19:48–50
Gu CQ, Xie CQ, Hu XY, Zhang WP, Bi DR, Cheng GF (2012) Cytokine gene expression in the livers of ducklings infected with duck hepatitis virus-1 JX strain. Poult Sci 91:583–591
Moebius U, Kober G, Griscelli AL, Hercend T, Meuer SC (1991) Expression of different CD8 isoforms on distinct human lymphocyte subpopulations. Eur J Immunol 21:1793–1800
Mak TW, Rahemtulla A, Schilham M, Koh DR, Fung-Leung WP (1992) Generation of mutant mice lacking surface expression of CD4 or CD8 by gene targeting. J Autoimmun 5:55–59
Nagarajan UM, O’Connell C, Rank RG (2004) Molecular characterization of guinea-pig (Cavia porcellus) CD8 alpha and CD8 beta cDNA. Tissue Antigens 63:184–189
Leahy DJ, Axel R, Hendrickson WA (1992) Crystal structure of a soluble form of the human T-cell coreceptor CD8 at 2.6 Å resolution. Cell 68:1145–1162
Gao GF, Tormo J, Gerth UC, Wyer JR, McMichael AJ, Stuart DI, Bell JI, Jones EY, Jakobsen BK (1997) Crystal structure of the complex between human CD8α and HLA-A2. Nature 387:630–634
Tanabe M, Karaki S, Takiguchi M, Nakauchi H (1992) Antigen recognition by the T-cell receptor is enhanced by CD8 alpha-chain binding to the alpha 3 domain of MHC class I molecules, not by signaling via the cytoplasmic domain of CD8 alpha. Int Immunol 4:147–152
Shaw AS, Chalupny J, Whitney JA, Hammond C, Amrein KE, Kavathas P, Sefton BM, Rose JK (1990) Short related sequences in the cytoplasmic domains of CD4 and CD8 mediate binding to the amino-terminal domain of the p56lck tyrosine protein kinase. Mol Cell Biol 10:1853–1862
Barber EK, Dasgupta JD, Schlossman SF, Trevillyan JM, Rudd CE (1989) The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci 86:3277–3281
Louise GD, Sham VN, Elizabeth MD (2009) The marsupial CD8 gene locus: molecular cloning and expression analysis of the alpha and beta sequences in the gray short-tailed opossum (Monodelphis domestica) and the tammar wallaby (Macropus eugenii). Vet Immunol Immunopathol 129:14–27
Simona P, Laura G, Francesco B (2009) Lymphocyte differentiation in sea bass thymus: CD4 and CD8-α gene expression studies. Fish Shellfish Immunol 27:50–56
Yue H, Huang X, Yang FL, Li MY, Fan GC, Ma L, Tang C (2008) Development of real-time RT-PCR for detecting the expression level of CD4 and CD8 mRNA in chicken. Acta Vet Zootech Sin 39:784–790
Hansen JD, Zapata AG (1998) Lymphocyte development in fish and amphibians. Immunol Rev 166:199–220
Zwollo P, Cole S, Bromage E, Kaattari S (2005) B-cell heterogeneity in the teleost kidney: evidence for a maturation gradient from anterior to posterior kidney. J Immunol 174:6608–6616
Davidson GA, Lin SH, Secombes CJ, Ellis AE (1997) Detection of specific and ‘constitutive’ antibody secreting cells in the gills, head kidney and peripheral blood leucocytes of dab (Limanda limanda). Vet Immunol Immunopathol 58:363–374
Anwarul Islam MD, Adrian O, Vladutiu MD, Theresa Donahue MT (2000) CD8 expression on B cells in chronic lymphocytic leukemia. Arch Pathol Lab Med 124:1361–1363
Giovanni Carulli MD, Alessandra S, Alessandra Marini MD (2009) Aberrant expression of CD8 in B-cell non-Hodgkin lymphoma. Am J Clin Pathol 132:186–190
Morimura T, Ohashi K, Kon Y, Hattori M, Sugimoto C, Onuma M (1996) Apoptosis and CD8-down-regulation in the thymus of chickens infected with Marek’s disease virus. Arch Virol 141:2243–2249
Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M (2010) KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res 38:355–360
Abdul Careem MF, Hunter BD (2007) Cytokine gene expression patterns associated with immunization against Marek’s disease in chickens. Vaccine 25:424–432
Acknowledgments
We are grateful to Prof. Peng DX and Dr. Chen SJ (Yangzhou University) for their suggestions and technical assistance. This work was supported financially by the National Natural Science Foundation of China (31101704), Natural Science Foundation of Jiangsu Province (BK20141275) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (2011-137).
Conflict of interest
The authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, Q., Chen, Y., Zhao, W.M. et al. The CD8α gene in duck (Anatidae): cloning, characterization, and expression during viral infection. Mol Biol Rep 42, 431–439 (2015). https://doi.org/10.1007/s11033-014-3784-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3784-3