Abstract
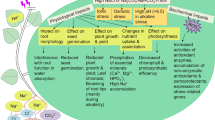
Transgenic rice plants co-expressing the Suaeda salsa SsNHX1 (vacuolar membrane Na+/H+ antiporter) and Arabidopsis AVP1 (vacuolar H+-PPase) showed enhanced salt tolerance during 3 d of 300 mM NaCl treatment under outdoor growth conditions. These transgenic rice seedlings also grew better on MS medium containing 150 mM NaCl compared to SsNHX1-transformed lines and non-transformed controls. Measurements on isolated vacuolar membrane vesicles derived from the salt stressed SsNHX1+AVP1-transgenic plants demonstrated that the vesicles had increased V-PPase hydrolytic activity in comparison with the Ss-transgenics and non-transgenics. Moreover the V-PPase activity was closely related to the development period of the SA-transgenic seedlings and markedly higher in 3-week-old seedlings than in 5-week-old seedlings. Statistic analysis indicated that the SA-transgenic rice plants contained relatively more ions with higher K+/Na+ ratio in their shoots compared to the SsNHX1-transformed lines upon salt treatment. Furthermore, these SA-transformants also exhibited relatively higher level of photosynthesis and root proton exportation capacity whereas reduced H2O2 generation in the same plants. In general, these results supported the hypothesis that simultaneous expression of the SsNHX1 and AVP1 conferred greater performance to the transgenic plants than that of the single SsNHX1.
Similar content being viewed by others
References
Allakhverdiev SI, Nishiyama Y, Suzuki I, Tasaka Y, Murata N (1999) Genetic engineering of the unsaturation of fatty acids in membrane lipids alters the tolerance of Synechocystis to salt stress. Proc Natl Acad Sci USA 96:5862–5867
Allakhverdiev SI, Sakamoto A, Nishiyama Y, Inaba M, Murata␣N (2000a) Ionic and osmotic effects of NaCl-induced␣inactivation of photosystems I and II in Synechococcus sp. Plant Physiol 123:1047–1056
Allakhverdiev SI, Sakamoto A, Nishiyama Y, Murata N (2000b) Inactivation of photosystems I and II in response to osmotic stress in Synechococcus: contribution of water channels. Plant Physiol 122:1201–1208
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285:1256–1258
Barklaz BJ, Zingarelli L, Blumwald E, Smith JAC (1995) Tonoplast Na+/H+ antiport activity and its energization by the vacuolar H+-ATPase in the halophytic plant Mesembryanthemum crystallinum. Plant Physiol 109:549–556
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Fukuda A, Nakamura A, Tagiri A, Tanaka H, Miyao A, Hirochika H, Tanaka Y (2004) Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol 45:149–159
Gao XH, Ren ZH, Zhao YX, Zhang H (2003) Overexpression of SOD2 increases salt tolerance of Arabidopsis. Plant Physiol 133:1873–1881
Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR (2001) Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA 98(20):11444–11449
Golldack D, Dietz KJ (2001) Salt-induced expression of the vacuolar H+-ATPase in the common ice plant is developmentally controlled and tissue specific. Plant Physiol 125(4):1643–1654
Greenway H, Munns R (1980) Mechanisms of salt tolerance in non-halophytes. Annu Rev Plant Physiol 31:149–190
Horie T, Schroede JI (2004) Sodium transporters in plants. Diverse genes and physiological functions. Plant Physiol 136:2457–2462
Jang IC, Nahm BH, Kim JK (1999) Subcellular targeting of green fluorescent protein to plastids in transgenic rice plants provides a high-level expression system. Mol Breed 5:453–461
Leigh RA, Gordon-Weeks R, Steele SH, Koren'kov VD (1994) The H(+)-pumping inorganic pyrophosphatase of the vacuolar membrane of higher plants. Symp Soc Exp Biol 48:61–75
Liu QQ, Zang JL, Wang ZY, Hong MM, Gu MH (1998) A highly efficient transformation system mediated by Agrobacterium tumefaciens in Rice (Oryza sativa L.). Acta Phytophysiol Sin 24(3):259–271
Lin ZF, Li SS, Lin GZ (1988) The accumulation of hydrogen peroxide in senescencing leaves and chloroplasts in relation to lipid peroxidation. Acta Phytophysiol Sin 14(1):16–22
Ma XL, Zhang Q, Shi HZ, Zhu JK, Zhao YX, Ma CL, Zhang H (2004) Molecular cloning and different expression of a vacuolar Na+/H+ antiporter gene in Suaeda salsa under salt stress. Biol Plant 48(2):219–225
Maathuis FJ, Filatov V, Herzyk P, Krijger GC, Axelsen KB, Chen S, Green BJ, Li Y, Madagan KL, Sanchez-Fernandez R, Forde BG, Palmgren MG, Rea PA, Williams LE, Sanders D, Amtmann A (2003) Transcriptome analysis of root transporters reveals participation of multiple gene families in the response to cation stress. Plant J 35(6):675–692
Maeshima M (1991) H(+)-translocating inorganic pyrophosphatase of plant vacuoles. Inhibition by Ca2+, stabilization by Mg2+ and immunological comparison with other inorganic pyrophosphatases. Eur J Biochem 196(1):11–17
Mittova V, Guy M, Tal M, Volokita M (2004) Salinity up-regulates the antioxidative system in root mitochondria and peroxisomes of the wild salt-tolerant tomato species Lycopersicon pennellii. J Exp Bot 55(399):1105–1113
Mohanty A, Kathuria H, Ferjani A, Sakamoto A, Mohanty P, Murata N, Tyagi AK (2002) Transgenics of an elite indica rice variety Pusa Basmati 1 harbouring the codA gene are␣highly tolerant to salt stress. Theor Appl Genet 106(1):51–57
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Niu X, Bressan RA, Hasegawa PM, Pardo JM (1995) Ion homeostasis in NaCl stress environments. Plant Physiol 109:735–742
Ohta M, Hayashi Y, Nakashima A, Hamada A, Tanaka A, Nakamura T, Hayakawa T (2002) Introduction of a Na+/H+antiporter gene from Atriplex gmelini confers salt tolerance to rice. FEBS Lett 532:279–282
Papageorgiou GC, Alygizaki-Zorba A, Ladas N, Murata N (1998) A method to probe the cytoplasmic osmolality and osmotic water and solute fluxes across the cell membrane of cyanobacteria with Chl a fluorescence: experiments with Synechococcus sp. PCC 7942. Physiol Plant 103:215–224
Pagis LY, Popova LG, Andreev IM, Balnokin YV (2003) Comparative characterization of the two primary pumps, H+-ATPase and Na+-ATPase, in the plasma membrane of the marine alga Tetraselmis viridis. Physiol Plant 118:514–522
Parks GE, Dietrich MA, Schumaker KS (2002) Increased vacuolar Na(+)/H(+) exchange activity in Salicornia bigelovii Torr. in response to NaCl. J Exp Bot 53(371):1055–1065
Qiu NW, Lu QT, Lu CM (2003) Photosynthesis, photosystem II efficiency and the xanthophyll cycle in the salt-adapted␣halophyte Atriplex centralasiatica. New Phytol 159:479–486
Ren ZH, Gao JP, Li LG, Cai XL, Huang W, Chao DY, Zhu MZ, Wang ZY, Luan S, Lin HX (2005) A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet 37(10):1141–6
Shaul O (2002) Magnesium transport and function in plants: the tip of the iceberg. Biometals 15:309–323
Sze H, Lia X, Palmgrenb MG (1999) Energization of plant cell membranes by H-pumping ATPases: regulation and biosynthesis. Plant Cell 11:677–690
Talarczyk A, Krzymowska M, Borucki W, Hennig J (2002) Effect of yeast CAT1 gene expression on response of tobacco plants to Tobacco Mosaic Virus infection. Plant Physiol 129:1032–1044
Vasekina AV, Yershov PV, Reshetova OS, Tikhonova TV, Lunin VG, Trofimova MS, Babakov AV (2005) Vacuolar Na+/H+ antiporter from barley: identification and response to salt stress. Biochemistry (Mosc) 70(1):100–107
Wang BS, Lüttge U, Rataj R (2001) Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa. J Exp Bot 52(365):2355–2365
Xue ZY, Zhi D, Xue GP, Zhang H, Zhao YX, Xia GM (2004) Enhanced salt tolerance of transgenic wheat (Tritivum aestivum L.) expressing a vacuolar Na+/H+ antiporter gene with improved grain yields in saline soils in the field and a reduced level of leaf Na+. Plant Sci 167:849–859
Yan F, Zhu Y, Müller C, Zörb C, Schubert S (2002) Adaptation of H+-pumping and plasma membrane H+ ATPase activity in Proteoid roots of White Lupin under phosphate deficiency. Plant Physiol 129:50–63
Yang T, Poovaiah BW (2002) Hydrogen peroxide homeostasis: Activation of plant catalase by calcium/calmodulin. Proc Natl Acad Sci USA 99(6):4097–4102
Zhang HX, Blumwald E (2001) Transgenic salt tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768
Zhang HX, Hodson J, Williams JP, Blumwald E (2001) Engineering salt tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci USA 98:12832–12836
Acknowledgements
This work is supported financially by the National High Technology Research and Development Program of China (863 Program No. 2002AA629080) and the National Key Fundamental Research Program of China (973 Program No. 2006CB100104).
Author information
Authors and Affiliations
Corresponding author
Additional information
Feng-Yun Zhao and Xue-Jie Zhang contributed equally to this work
Rights and permissions
About this article
Cite this article
Zhao, FY., Zhang, XJ., Li, PH. et al. Co-expression of the Suaeda salsa SsNHX1 and Arabidopsis AVP1 confer greater salt tolerance to transgenic rice than the single SsNHX1 . Mol Breeding 17, 341–353 (2006). https://doi.org/10.1007/s11032-006-9005-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11032-006-9005-6