Abstract
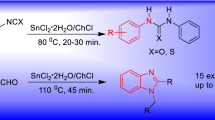
An efficient and green synthesis of pyrazolyl-2,4-thiazolidinediones/pyrazolyl-2-iminothiazolidine-4-ones 7(a–j) has been developed using urea/thiourea as catalyst. Two methods (A and B) have been introduced for the synthesis of these compounds. Method A performed well for the condensation of pyrazole-4-carboxaldehydes 4(a–e) with 2-iminothiazolidin-4-one 5a and with 2,4-thiazolidinedione 5b at 110 \(^{\circ }\hbox {C}\) for 16–20 min to furnish (Z)-5-((1,3-diphenyl-1H-pyrazol-4-yl)methylene)thiazolidine-2,4-diones 7(a–j) in excellent yields. In method B, the pyrazole-4-carboxaldehydes 4(a–e) were condensed with urea/thiourea at 110 \(^{\circ }\hbox {C}\) for 10 min to give key intermediates 6(a–j) which were later condensed with 5a and 5b to afford 7(a–j) via the elimination of urea/thiourea rather than the formation of a Biginelli product. These two protocols are inexpensive, eco-friendly and high yielding to provide the final products.
Graphical Abstract
The synthesis of compound 7f









Similar content being viewed by others
References
Bekhit AA, Ashour HM, Bekhit AD, Bekhit SA (2009) Synthesis and biological evaluation of novel pyrazole derivatives as anti-inflammatory antimicrobial agents. Med Chem 5:103–117. doi:10.2174/157340609787582936
Manojkumar P, Ravi TK, Subbuchettiar G (2009) Synthesis of coumarin heterocyclic derivatives with antioxidant activity and in vitro cytotoxic activity against tumour cells. Acta Pharm 59:159–170. doi:10.2478/v10007-009-0018-7
Sauzem PD, Santann SG, Machado P, Duarte MM, Ferreira J, Mello CF, Beck P, Bonacorso HG, Zanatta N, Martins MA, Rubin MA (2009) Effect of 5-trifluoromethyl-4,5-dihydro-1H-pyrazoles on chronic inflammatory pain model in rats. Eur J Pharmacol 616:91–100. doi:10.1016/j.ejphar.2009.06.008
Damljanovic I, Vukicevic M, Radulovic N, Palic R, Ellmerer E, Ratkovic Z, Joksovic MD, Vukicevic RD (2009) Synthesis and antimicrobial activity of some new pyrazole derivatives containing a ferrocene unit. Bioorg Med Chem Lett 19:1093–1096. doi:10.1016/j.bmcl.2009.01.006
Gao HM, Hong JS (2008) Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol 29:357–365. doi:10.1016/j.it.2008.05.002
Carroll RT, Dluzen DE, Stinnett H, Awalex PS, Funk MO, Geldenhuys W (2011) Structure-activity relationship and docking studies of thiazolidinedione-type compounds with monoamine oxidase B. Bioorg Med Chem Lett 21:4798–4803. doi:10.1016/j.bmcl.2011.06.060
Liu XF, Zheng CJ, Sun LP, Piao HR (2011) Synthesis of new chalcone derivatives bearing 2,4-thiazolidinedione and benzoic acid moieties as potential anti-bacterial agents. Eur J Med Chem 46:3469–3473. doi:10.1016/j.ejmech.2011.05.012
Sunduru N, Srivastava K, Rajakumar S, Puri SK, Saxena JK, Chauhan PMS (2009) Synthesis of novel thiourea, thiazolidinedione and thioparabanic acid derivatives of 4-aminoquinoline as potent antimalarials. Bioorg Med Chem Lett 19:2570–2573. doi:10.1016/j.bmcl.2009.03.026
Tomasic T, Masic LP (2009) Rhodanine as a privileged scaffold in drug discovery. Curr Med Chem 16:1596–1629. doi:10.2174/092986709788186200
Irvine MW, Patrick GL, Kewney J, Hastings SF, MacKenzie S (2008) Rhodanine derivatives as novel inhibitors of PDE4. Bioorg Med Chem Lett 18:2032–2037. doi:10.1016/j.bmcl.2008.01.117
Komatsu T, Hirano T, Songkram C, Kagechika EKH (2007) Novel thyroid hormone receptor antagonists with an N-alkylated diphenylamine skeleton. Bioorg Med Chem 15:3115–31126. doi:10.1016/j.bmc.2007.02.053
Prakash O, Deepak KA, Lohan P, Hussain K, Sanjiv A, Sharma C, Aneja KR (2012) Synthesis and antimicrobial activity of 5-((3-aryl-1-phenyl-1H-pyrazol-4-yl)methylene)thiazolidine-2,4-diones. Med Chem Res 21:2961–2968. doi:10.1186/2191-2858-1-15
Amal M, Youssef M, Sydney W, Erika BV, El-Ashmawy IM, Andis K (2010) Synthesis and biological evaluation of novel pyrazolyl-2,4-thiazolidinediones as anti-inflammatory and neuroprotective agents. Bioorg Med Chem 18:2019–2028. doi:10.1016/j.bmc.2010.01.021
Jawale DV, Pratap UR, Lingampalle DL, Mane RA (2011) Dicationic ionic liquid mediated synthesis of 5-arylidine-2,4-thiazolidinediones. Chin J Chem 29:942–946. doi:10.1002/cjoc.201190192
Vachal P, Jacobsen EN (2002) Structure-based analysis and optimization of a highly enantio selective catalyst for the strecker reaction. J Am Chem Soc 124:10012–10014. doi:10.1021/ja027246j
Cozzi F (2006) Immobilization of organic catalysts: when, why, and how. Adv Synth Catal 348:1367–1390. doi:10.1002/adsc.200606096
Curran DP, Kuo LH (1995) Acceleration of a dipolar Claisen rearrangement by hydrogen bonding to a soluble diaryl urea. Tetrahedron Lett 36:6647–6650. doi:10.1016/00404-0399(50)1394-W
Okino T, Hoashi Y, Takemoto Y (2003) Thiourea-catalyzed nucleophilic addition of TMSCN and ketene silyl acetals to nitrones and aldehydes. Tetrahedron Lett 44:2817–2821. doi:10.1016/S0040-4039(03)00433-7
Maher DJ, Connon SJ (2004) Acceleration of the DABCO-promoted Baylis–Hillman reaction using a recoverable H-bonding organocatalyst. Tetrahedron Lett 45:1301–1305. doi:10.1016/j.tetlet.2003.11.062
Li JT, Li YW, Song YL, Chen GF (2012) Improved synthesis of 2,2\(^\prime \)-arylmethylenebis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) derivatives catalyzed by urea under ultrasound. Ultrason Sonochem 19:1–4. doi:10.1016/j.ultsonch.2011.05.001
Kira MA, Abdelraeman MO, Gadalla KZ (1969) The Vilsmeier–Haack reaction-III cyclization of hydrazones to pyrazoles. Tetrahedron Lett 2:109–110. doi:10.1016/S0040-4039(01)88217-4
Prashantha BR, Adhikary LJ (2006) Microwave induced synthesis of the thiazolidine-2,4-dione motif and the efficient solvent free-solid phase parallel syntheses of 5-benzylidene-thiazolidine-2,4-dione and 5-benzylidene-2-thioxo-thiazolidine-4-one compounds. J Heterocycl Chem 43:897–903. doi:10.1002/jhet.5570430413
Momos Y, Meguro K, Hithosi I, Hatanaka C, Satoru O, Shoda T (1991) Studies on antidiabetic agents. X. Synthesis and biological activities of pioglitazone and related compounds. Chem Pharm Bull 39:1440–1445. doi:10.1248/cpb.39.1440
Acknowledgements
The author are indebted to University Grants Commission, Govt. of India, New Delhi, for providing financial support to one of them (K.V.S) in the form of UGC-SRF. They are also thankful to the authorities of Jawaharlal Nehru Technological University Hyderabad for providing the laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Konkala, V.S., Dubey, P.K. Urea/thiourea: efficient, inexpensive and reusable catalysts for the synthesis of pyrazole derivatives with 2-iminothiazolidin-4-one and 2,4-thiazolidinediones under solvent-free conditions. Mol Divers 21, 283–291 (2017). https://doi.org/10.1007/s11030-016-9719-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-016-9719-2