Abstract
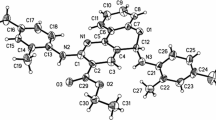
A facile and efficient synthesis of a library of novel chromeno[3,2-\(c\)]pyridines has been achieved from the reaction of various 3,5-((\(E\))-arylidene)-1-alkylpiperidin-4-ones and cyclic 1,3-diketones. The reaction presumably occurred via tandem Michael addition–intramolecular \(O\)-cyclization–elimination sequence in a single operation.







Similar content being viewed by others
References
Nunez-Vergara LJ, Squella JA, Navarrete-Encina PA, Vicente-Garcia E, Preciado S, Lavilla R (2011) Chromenopyridines: promising scaffolds for medicinal and biological chemistry. Curr Med Chem 18:4761–4785. doi:10.2174/092986711797535272
Evdokimov NM, Kireev AS, Yakovenko AA, Antipin MY, Magedov IV, Kornienko (2007) One-step synthesis of heterocyclic privileged medicinal scaffolds by a multicomponent reaction of malononitrile with aldehydes and thiols. J Org Chem 72:3443–3453. doi:10.1021/jo070114u
Anderson DR, Hegde S, Reinhard E, Gomez L, Vernier WF, Lee L, Liu S, Sambandam A, Snider PA, Masih L (2005) Aminocyanopyridine inhibitors of mitogen activated protein kinase-activated protein kinase 2 (MK-2). Bioorg Med Chem Lett 15:1587–1590. doi:10.1016/j.bmcl.2005.01.067
Unangst PC, Capiris T, Connor DT, Heffner TG, MacKenzie RG, Miller SR, Pugsley TA, Wise LD (1997) Chromeno[3,4-\(c\)]pyridin-5-ones: selective human dopamine D4 receptor antagonists as potential antipsychotic agents. J Med Chem 40:2688–2693. doi:10.1021/jm970170v
Bristol JA, Gold EH, Gross I, Lovey RG, Long JF (1981) Gastric antisecretory agents. Antisecretory activity of 9-[(aminoalkyl)thio]-9\(H\)-xanthenes and 5-[(aminoalkyl)thio]-5\(H\)-[l]benzopyrano[2,3-\(b\)]pyridines. J Med Chem 24:1010–1013. doi:10.1021/jm00140a020
Kolokythas G, Pouli N, Marakos P, Pratsinis H, Kletsas D (2006) Design, synthesis and antiproliferative activity of some new azapyranoxanthenone aminoderivatives. Eur J Med Chem 41:71–79. doi:10.1016/j.ejmech.2005.10.011
Azuine MA, Tokuda H, Takayasu J, Enjyo F, Mukainaka T, Konoshima T, Nishino H, Kapadia GJ (2004) Cancer chemopreventive effect of phenothiazines and related tri-heterocyclic analogues in the 12-\(O\)-tetradecanoylphorbol-13-acetate promoted Epstein-Barr virus early antigen activation and the mouse skin two-stage carcinogenesis models. Pharmacol Res 49:161–169. doi:10.1016/j.phrs.2003.07.014
Srivastava SK, Tripathi RP, Ramachandran R (2005) DNA: replication, repair, and recombination: NAD\(^{+}\)-dependent DNA ligase (Rv3014c) from mycobacterium tuberculosis crystal structure of the adenylation domain and identification of novel inhibitors. J Biol Chem 280:30273–30281. doi:10.1074/jbc.M503780200
Brotz-Oesterhelt H, Knezevic I, Bartel S, Lampe T, Warnecke-Eberz U, Ziegelbauer K, Habich D, Labischinski H (2003) DNA: replication, repair, and recombination: specific and potent inhibition of NAD-dependent DNA ligase by pyridochromanones. J Biol Chem 278:39435–39442. doi:10.1074/jbc.M306479200
Ukawa K, Ishiguro T, Kuriki H, Nohara A (1985) Synthesis of the metabolites and degradation products of 2-amino-7-isopropyl-5-oxo-5\(H\)-[1]benzopyrano[2,3-\(b\)]pyridine-3-carboxyl-ic acid (Amoxanox). Chem Pharma Bull 33:4432–4437
Yetra SR, Roy T, Bhunia A, Porwal D, Biju AT (2014) Synthesis of functionalized coumarins and quinolinones by NHC catalyzed annulation of modified enals with heterocyclic C-H acids. J Org Chem 79:4245–4251. doi:10.1021/jo500693h
Elinson MN, Gorbunov SV, Vereshchagin AN, Nasybullin RF, Goloveshkin AS, Bushmarinov IS, Egorov MP (2014) Chemical and electrocatalytic cascade cyclization of salicylaldehyde with three molecules of malononitrile:‘one-pot’ simple and efficient way to the chromeno[2,3-\(b\)]pyridine scaffold. Tetrahedron 70:8559–8563. doi:10.1016/j.tet.2014.09.066
Gan H-F, Cao W-W, Fang Z, Li X, Tang S-G, Guo K (2014) Efficient synthesis of chromenopyridine and chromene via MCRs. Chin Chem Lett 25:1357–1362. doi:10.1016/j.cclet.2014.05.008
Mao Z, Lin A, Shi Y, Mao H, Li W, Cheng Y, Zhu C (2013) Chiral tertiary amine thiourea-catalyzed asymmetric inverse electron-demand Diels-Alder reaction of chromone heterodienes using 3vinylindoles as dienophiles. J Org Chem 78:10233–10239. doi:10.1021/jo401592w
Ammar YA, El-Gaby MSA, Salem MA (2013) Cyanoacetanilides intermediates in heterocyclic synthesis. Part 6: Preparation of some hitherto unknown 2-oxopyridine, bipyridine, isoquinoline and chromeno[3,4-\(c\)]pyridine containing sulfonamide moiety (Article in press). Arabian J Chem 7(5):615–622. doi:10.1016/j.arabjc.2013.11.026
Mishra A, Rastogi N, Batra S (2012) 2-(\(N\)-Allylaminomethyl)cinnamaldehydes as substrates for syntheses of aza-polycycles via intramolecular cycloaddition reactions. Tetrahedron 68:2146–2154. doi:10.1016/j.tet.2012.01.016
Yan J, Cheng M, Hu F, Hu Y (2012) Direct synthesis of functional azaxanthones by using a domino three-component reaction. Org Lett 14:3206–3209. doi:10.1021/ol3013099
Mohammadzadeh I, Sheibani H (2012) A convenient one-pot synthesis of new chromeno[3,4-\(c\)]chromene and chromeno[3,4-\(c\)]pyridine derivatives in the presence of high surface area of magnesium oxide. Chin Chem Lett 23:1327–1330. doi:10.1016/j.cclet.2012.10.007
Venkati M, Reddy SS, Swamy GYSK, Ravikumar K, Krupadanam GLD (2012) Synthesis of 5-aryl-2-piperidino-5\(H\)-chromeno[3,4-\(c\)]pyridine-1-carbonitriles. Arkivoc vi:355–364 (12–7010IP)
Voskressensky LG, Kulikova LN, Gozun SV, Khrustalev VN, Borisova TN, Listratova AV, Ovcharov MV, Varlamov AV (2011) The reaction of tetrahydrochromeno[3,4-\(c\)]pyridines with activated alkynes. The first synthesis of tetrahydrochromeno[4,3-\(d\)]azocines. Tetrahedron Lett 52:4189–4191. doi:10.1016/j.tetlet.2011.06.012
Sriram D, Yogeeswari P, Banerjee M, Bhat P, Gadhwal S (2010) Discovery of novel antitubercular 2,10-dihydro-4a\(H\)-chromeno-[3,2-\(c\)]pyridin-3-yl derivatives. Eur J Med Chem 45:120–123. doi:10.1016/j.ejmech.2009.09.033
Plaskon AS, Ryabukhin SV, Volochnyuk DM, Gavrilenko KS, Shivanyuk AN, Tolmachev AA (2008) Synthesis of quinolines from 3-formylchromone. J Org Chem 73:6010–6013. doi:10.1021/jo800950y
Sosnovskikh VYa, Irgashev RA, Kodess MI (2008) One-pot three-component reaction of 3-(polyfluoroacyl)chromones with active methylene compounds and ammonium acetate: regioselective synthesis of novel R\(^{F}\)-containing nicotinic acid derivatives. Tetrahedron 64:2997–3004. doi:10.1016/j.tet.2008.01.076
Hai-Bin T, Duan-Zhi Y, Lan Z, Li-Hua W, Chun-Fu Z, Ming-Wei W, Chun-Ying W, Gu-Cai L, Yong-Xian W (2005) Dopamine D4 receptor antagonist 3-(4-[\(^{18}\)F]fluorobenzyl)-8-methoxy-1,2,3,4-tetrahydrochromeno[3,4-\(c\)]pyridin-5-one([\(^{18}\)F]FMTP): radiosynthesis and in vivo characterization in rats. Appl Radiat Isot 63:333–342. doi:10.1016/j.apradiso.2005.04.004
Zhang M-R, Haradahira T, Maeda J, Okauchi T, Kawabe K, Kida T, Obayashi S, Suzuki K, Suhara T (2002) Synthesis and evaluation of 3-(4-chlorobenzyl)-8-[\(^{11}\)C]methoxy-1,2,3,4-tetrahydrochromeno[3,4-\(c\)]pyridin-5-one: a PET tracer for imaging \(\text{ sigma }_{ 1}\) receptors. Nucl Med Biol 29:469–476. doi:10.1016/S0969-8051(02)00293-7
Samanta S, Gupta AD, Mondal R, Mallik AK (2013) A simple synthesis of \(E\)-9-aryl-5-arylidene-1-oxo-1,2,3,4,5,6,7,8-octahydroxanthenes and their lower analogues from \(E\),\(E-\alpha \),\(\alpha \prime \)-diarylidenecycloalkanones. J. Chem. Res. 125:737–743
Tietz LF (1996) Domino reactions in organic synthesis. Chem Rev 96:115–136. doi:10.1021/cr950027e
Tietze LF, Rackelmann N (2004) Domino reactions in the synthesis of heterocyclic natural products and analogs. Pure Appl Chem 76:1967–1983. doi:10.1351/pac200476111967
Tietze LF, Brasche G, Gericke KM (2006) Domino reactions in organic synthesis. Wiley, Weinheim. doi:10.1002/9783527609925.fmatter
Nicolaou KC, Edmonds DJ, Bulger PG (2006) Cascade reactions in total synthesis. Angew Chem Int Ed 45:7134–7186. doi:10.1002/anie.200601872
Toure BB, Hall DG (2009) Natural product synthesis using multicomponent reaction strategies. Chem Rev 109:4439–4486. doi:10.1021/cr800296p
LaPorte MG, Goodell JR, Tsegay S, Wipf P (2013) Diversity-oriented synthesis: basics and applications in organic synthesis, drug discovery, and chemical biology. In: Trabocchi A (ed) Domino reactions in library synthesis. Wiley, Hoboken
Tietze LF (ed) (2014) Domino reactions: concepts for efficient organic synthesis. Wiley, Weinheim
Xie YJ, Sun J, Yan CG (2014) Domino reactions of vinyl malononitriles with 3phenacylideneoxindoles for efficient synthesis of functionalized spirocyclic oxindoles. ACS Comb Sci 16:271–280. doi:10.1021/co500006c
Marek I, Minko Y, Pasco M, Mejuch T, Gilboa N, Chechik H, Das JP (2014) All-carbon quaternary stereogenic centers in acyclic systems through the creation of several C-C bonds per chemical step. J Am Chem Soc 136:2682–2694. doi:10.1021/ja410424g
Jiang B, Fan W, Sun MY, Ye Q, Wang SL, Tu SJ, Li G (2014) Domino reaction of arylglyoxals with pyrazol-5-amines: selective access to pyrazolo-fused 1,7-naphthyridines, 1,3-diazocanes, and pyrroles. J Org Chem 79:5258–5268. doi:10.1021/jo500823z
Chen XB, Wang XY, Zhu DD, Yan SJ, Lin J (2014) Three-component domino reaction synthesis of highly functionalized bicyclic pyrrole derivatives. Tetrahedron 70:1047–1054. doi:10.1016/j.tet.2013.12.062
Kashiwagi T, Kotani S, Nakajima M, Sugiura M (2014) Diastereoselective synthesis of 1,3-diamines by a domino reaction of imines, enamines, and trichlorosilane. Tetrahedron Lett 55:1924–1926. doi:10.1016/j.tetlet.2014.01.152
Sivakumar S, Ranjith Kumar R (2014) Domino Knoevenagel condensation/Aza-Ene addition/\(N\)-cyclisation route to functionalized imidazo[1,2-\(a\)]pyridines and pyrido[1,2-\(a\)]pyrimidines. Asian J Org Chem 3:974–983. doi:10.1002/ajoc.201402100
Sivakumar S, Kanchithalaivan S, Ranjith Kumar R (2013) A one-pot three-component domino protocol for the synthesis of penta-subsituted 4\(H\)-pyrans. RSC Adv 3:13357–13364. doi:10.1039/C3RA41510D
Jayachandran V, Ranjith Kumar R, Ali MA, Choon TS (2013) A one-pot domino synthesis and discovery of highly functionalized dihydrobenzo[\(b\)]thiophenes as AChE inhibitors. Bioorg Med Chem Lett 23:2101–2105. doi:10.1016/j.bmcl.2013.01.122
Maharani S, Ranjith Kumar R (2013) ‘On-water’ one-pot pseudo four-component domino protocol for the synthesis of novel benzo[\(a\)]cyclooctenes. Tetrahedron Lett 54:4800–4802. doi:10.1016/j.tetlet.2013.06.139
Kanchithalaivan S, Sivakumar S, Ranjith Kumar S, Elumalai P, Ahmed QN, Padala AK (2013) Four-component domino strategy for the combinatorial synthesis of novel 1,4-dihydropyrano[2,3-\(c\)]pyrazol-6-amines. ACS Comb Sci 15:631–638. doi:10.1021/co4000997
Ranjith Kumar R, Perumal S, Senthilkumar P, Yogeeswari P, Sriram D (2008) Discovery of antimycobacterial spiro-piperidin-4-ones: an atom economic, stereoselective synthesis and biological intervention. J Med Chem 51:5731–5735. doi:10.1021/jm800545k
Acknowledgments
RRK thanks the University Grants Commission, New Delhi for funds through Major Research Project F. No. 42-242/2013 (SR) and Department of Science and Technology, New Delhi for funds under (i) IRHPA program for the high resolution NMR facility in the Department and (ii) PURSE program. RVS thanks the University Grants Commission, New Delhi for research fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sumesh, R.V., Malathi, A. & Ranjith Kumar, R. A facile tandem Michael addition/O-cyclization/elimination route to novel chromeno[3,2-c]pyridines. Mol Divers 19, 233–249 (2015). https://doi.org/10.1007/s11030-015-9576-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-015-9576-4