Abstract
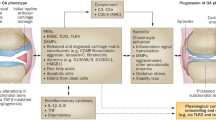
Osteoarthritis (OA) is a chronic disease that degrades the joints and is often associated with increasing age and obesity. The two most common sites of OA in adults are the knee and hip joints. Increased mechanical stress on the joint from obesity can cause the articular cartilage to degrade and release damage-associated molecular patterns (DAMPs). These DAMPs are involved in various molecular pathways that interact with nuclear factor-kappa B and result in the transcription of inflammatory cytokines and activation of matrix metalloproteinases that progressively destroy cartilage. This review focuses on the interactions and contribution to the pathogenesis and progression of OA through the DAMPs: high-mobility group box 1 (HMGB-1), the receptor for advanced glycation end-products (RAGE), the alarmin proteins S100A8 and S100A9, and heparan sulfate. HMGB-1 is released from damaged or necrotic cells and interacts with toll-like receptors (TLRs) and RAGE to induce inflammatory signals, as well as behave as an inflammatory cytokine to activate innate immune cells. RAGE interacts with HMGB-1, advanced glycation end-products, and innate immune cells to increase local inflammation. The alarmin proteins are released following cell damage and interact through TLRs to increase local inflammation and cartilage degradation. Heparan sulfate has been shown to facilitate the binding of HMGB-1 to RAGE and could play a role in the progression of OA. Targeting these DAMPs may be the potential therapeutic strategies for the treatment of OA.


Similar content being viewed by others
References
Yucesoy B, Charles LE, Baker B, Burchfiel CM (2015) Occupational and genetic risk factors for osteoarthritis: a review. Work 50(2):261–273. doi:10.3233/WOR-131739
Sokolove J, Lepus CM (2013) Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 5(2):77–94. doi:10.1177/1759720X12467868
Ulloa L, Batliwalla FM, Andersson U, Gregersen PK, Tracey KJ (2003) High mobility group box chromosomal protein 1 as a nuclear protein, cytokine, and potential therapeutic target in arthritis. Arthritis Rheum 48(4):876–881. doi:10.1002/art.10854
Chayanupatkul M, Honsawek S (2010) Soluble receptor for advanced glycation end products (sRAGE) in plasma and synovial fluid is inversely associated with disease severity of knee osteoarthritis. Clin Biochem 43(13–14):1133–1137. doi:10.1016/j.clinbiochem.2010.07.007
Schelbergen RF, Blom AB, van den Bosch MH, Slöetjes A, Abdollahi-Roodsaz S, Schreurs BW et al (2012) Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on toll-like receptor 4. Arthritis Rheum 64(5):1477–1487. doi:10.1002/art.33495
Zreiqat H, Belluoccio D, Smith MM, Wilson R, Rowley LA, Jones K et al (2010) S100A8 and S100A9 in experimental osteoarthritis. Arthritis Res Ther 12(1):R16. doi:10.1186/ar2917
Vincent HK, Heywood K, Connelley J, Hurley RW (2012) Weight loss and obesity in the treatment and prevention of osteoarthritis. Am Acad Phys Med Rehabil 4(50):S59–S67. doi:10.1016/j.pmrj.2012.01.005.Weight
Turkiewicz A, Petersson IF, Björk J, Hawker G, Dahlberg LE, Lohmander LS, Englund M (2014) Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarthr Cartil 22(11):1826–1832. doi:10.1016/j.joca.2014.07.015
Favre J, Erhart-Hledik JC, Chehab EF, Andriacchi TP (2016) Baseline ambulatory knee kinematics are associated with changes in cartilage thickness in osteoarthritic patients over 5 years. J Biomech. doi:10.1016/j.jbiomech.2016.04.029
Varady NH, Grodzinsky AJ (2016) Osteoarthritis year in review 2015: mechanics. Osteoarthr Cartil 24(1):27–35. doi:10.1016/j.joca.2015.08.018
Harris EC, Coggon D (2016) Hip osteoarthritis and work. Best Pract Res Clin Rheumatol 29(3):462–482. doi:10.1016/j.berh.2015.04.015
Wen L, Kang JH, Yim YR, Kim JE, Lee JW, Lee KE et al (2016) Associations between body composition measurements of obesity and radiographic osteoarthritis in older adults: data from the Dong-gu Study. BMC Musculoskelet Disord 17(1):192. doi:10.1186/s12891-016-1040-9
Goldring MB (2012) Articular cartilage degradation in osteoarthritis. HSS J 8(1):7–9. doi:10.1007/s11420-011-9250-z
Yu L, Wang L, Chen S (2010) Endogenous toll-like receptor ligands and their biological significance. J Cell Mol Med 14(11):2592–2603. doi:10.1111/j.1582-4934.2010.01127.x
Larkin DJ, Kartchner JZ, Doxey AS, Hollis WR, Rees JL, Wilhelm SK et al (2013) Inflammatory markers associated with osteoarthritis after destabilization surgery in young mice with and without receptor for advanced glycation end-products (RAGE). Front Physiol 4:121. doi:10.3389/fphys.2013.00121
Terada C, Yoshida A, Nasu Y, Mori S, Tomono Y, Tanaka M et al (2011) Gene expression and localization of high-mobility group box chromosomal protein-1 (HMGB-1) in human osteoarthritic cartilage. Acta Med Okayama 65(6):369–377
Hamada T, Torikai M, Kuwazuru A, Tanaka M, Horai N, Fukuda T et al (2008) Extracellular high mobility group box chromosomal protein 1 is a coupling factor for hypoxia and inflammation in arthritis. Arthritis Rheum 58(9):2675–2685. doi:10.1002/art.23729
Heinola T, Kouri VP, Clarijs P, Ciferska H, Sukura A, Salo J, Konttinen YT (2010) High mobility group box-1 (HMGB-1) in Osteoarthritic cartilage. Clin Exp Rheumatol 28(4):511–518
Liu-Bryan R (2013) Synovium and the innate inflammatory network in osteoarthritis progression topical collection on osteoarthritis. Curr Rheumatol Rep. doi:10.1007/s11926-013-0323-5
Liu-Bryan R, Terkeltaub R (2010) Chondrocyte innate immune myeloid differentiation factor 88-dependent signaling drives procatabolic effects of the endogenous toll-like receptor 2/toll-like receptor 4 ligands low molecular weight hyaluronan and high mobility group box chromosomal protein. Arthritis Rheum 62(7):2004–2012. doi:10.1002/art.27475
Loeser RF, Yammani RR, Carlson CS, Chen H, Cole A, Im HJ, Bursch LS, Yan SD (2005) Articular chondrocytes express the receptor for advanced glycation end products: potential role in osteoarthritis. Arthritis Rheum 52(8):2376–2385. doi:10.1002/art.21199
Hou CH, Fong YC, Tang CH (2011) HMGB-1 induces IL-6 production in human synovial fibroblasts through c-Src, Akt and NF-κB pathways. J Cell Physiol 226(8):2006–2015. doi:10.1002/jcp.22541
Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H et al (2006) The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther 8(3):R69. doi:10.1186/ar1939
van Lent PL, Blom AB, Schelbergen RF, Slöetjes A, Lafeber FP, Lems WF et al (2012) Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum 64(5):1466–1476. doi:10.1002/art.34315
Park PW, Reizes O, Bernfield M (2000) Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. J Biol Chem 275(39):29923–29926. doi:10.1074/jbc.R000008200
Korb-Pap A, Stratis A, Mühlenberg K, Niederreiter B, Hayer S, Echtermeyer F et al (2012) Early structural changes in cartilage and bone are required for the attachment and invasion of inflamed synovial tissue during destructive inflammatory arthritis. Ann Rheum Dis 71:1004–1011. doi:10.1136/annrheumdis-2011-200386
Xu D, Young J, Song D, Esko JD (2011) Heparan sulfate is essential for high mobility group protein 1 (HMGB1) signaling by the receptor for advanced glycation end products (RAGE). J Biol Chem 286(48):41736–41744. doi:10.1074/jbc.M111.299685
Cucchiarini M, Henrionnet C, Mainard D, Pinzano A, Madry H (2015) New trends in articular cartilage repair. J Exp Orthop 2(1):8. doi:10.1186/s40634-015-0026-0
Barker T, Henriksen VT, Rogers VE, Aguirre D, Trawick RH, Lynn Rasmussen G, Momberger NG (2014) Vitamin D deficiency associates with γ-tocopherol and quadriceps weakness but not inflammatory cytokines in subjects with knee osteoarthritis. Redox Biol 2(1):466–474. doi:10.1016/j.redox.2014.01.024
Heidari B, Heidari P, Hajian-Tilaki K (2011) Association between serum vitamin D deficiency and knee osteoarthritis. Int Orthop 35(11):1627–1631. doi:10.1007/s00264-010-1186-2
Heidari B, Javadian Y, Babaei M, Yousef-Ghahari B. (2015) Restorative effect of Vitamin D deficiency on knee pain and quadriceps muscle strength in knee osteoarthritis. Acta Med Iran 53(8):466–770. http://www.ncbi.nlm.nih.gov/pubmed/26545990
Rai V, Dietz NE, Dilisio MF, Radwan MM, Agrawal DK (2016) Vitamin D attenuates inflammation, fatty infiltration, and cartilage loss in the knee of hyperlipidemic microswine. Arthritis Res Ther. doi:10.1186/s13075-016-1099-6
Lee DE, Trowbridge RM, Ayoub NT, Agrawal DK (2015) High-mobility group box protein-1, matrix metalloproteinases, and Vitamin D in keloids and hypertrophic scars. Plast Reconstr Surg Glob Open 3(6):e425. doi:10.1097/GOX.0000000000000391
Oh B, Lee M (2014) Combined delivery of HMGB-1 box A peptide and S1PLyase siRNA in animal models of acute lung injury. J Control Release 175:25–35. doi:10.1016/j.jconrel.2013.12.008
Girard JP (2007) A direct inhibitor of HMGB1 cytokine. Chem Biol 14(4):345–347. doi:10.1016/j.chembiol.2007.04.001
Seol D, McCabe DJ, Choe H, Zheng H, Yu Y, Jang K et al (2012) Chondrogenic progenitor cells respond to cartilage injury. Arthritis Rheum 64(11):3626–3637. doi:10.1002/art.34613
Kim W, Hudson BI, Moser B, Guo J, Rong LL, Lu Y et al (2005) Receptor for advanced glycation end products and its ligands. Ann N Y Acad Sci 561:553–561. doi:10.1196/annals.1338.063
Shembade N, Ma A, Harhaj EW (2010) Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327(5969):1135–1139. doi:10.1126/science.1182364
Hensor EMA, Dube B, Kingsbury SR, Tennant A, Conaghan PG (2015) Toward a clinical definition of early osteoarthritis: onset of patient-reported knee pain begins on stairs. Data from the osteoarthritis initiative. Arthritis Care Res 67(1):40–47. doi:10.1002/acr.22418
Vincent HK, Heywood K, Connelly J, Hurley RW (2012) Obesity and weight loss in the treatment and prevention of osteoarthritis. PMR 4(5 Suppl):S59–S67. doi:10.1016/j.pmrj.2012.01.005
Rai V, Dilisio MF, Dietz NE, Agrawal DK (2017) Recent strategies in cartilage repair: a systemic review of the scaffold development and tissue engineering. J Biomed Mater Res A. doi:10.1002/jbm.a.36087
Funding
This work was supported by research Grants R01 HL112597, R01 HL116042, and R01 HL120659 to DK Agrawal from the National Heart, Lung and Blood Institute, National Institutes of Health, USA. The content of this review article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
As the corresponding author, I declare that this manuscript is original; that the article does not infringe upon any copyright or other proprietary right of any third party; that neither the text nor the data have been reported or published previously. All the authors have no conflict of interest and have read the journal’s authorship statement.
Rights and permissions
About this article
Cite this article
Rosenberg, J.H., Rai, V., Dilisio, M.F. et al. Damage-associated molecular patterns in the pathogenesis of osteoarthritis: potentially novel therapeutic targets. Mol Cell Biochem 434, 171–179 (2017). https://doi.org/10.1007/s11010-017-3047-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3047-4