Abstract
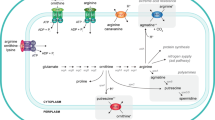
l-arginine (l-Arg) uptake is mediated by members of cationic amino acid transporter (CAT) family and may coincide with the induction of nitric oxide synthases (NOS). The present study was conducted to investigate the extracellular concentrations of l-Arg regulating the CAT-1, CAT-4 and inducible NOS (iNOS) in chick intestinal epithelial cells. The cells were cultured for 4 days in Arg-free Dulbecco’s modified Eagle’s medium containing 10, 100, 200, 400, or 600 μM l-Arg. Cell viability, nitric oxide (NO) concentrations, uptake and metabolism of l-[3H]-Arg as well as expression of CAT-1, CAT-4, and iNOS were determined. Our results showed that l-Arg enhances cell growth with a maximal response at 10–400 μM. Addition of 100, 200, or 400 μM l-Arg increased the l-[3H]-Arg uptake, which was associated with greater conversion of l-[3H]-citrulline and NO production in comparison with 10 μM l-Arg group. Increasing extracellular concentrations of l-Arg from 10 to 400 μM dose dependently increased the levels of CAT-1 mRNA and protein, while no effect on CAT-4 mRNA abundance was found. Furthermore, supplementation of 100, 200, or 400 μM l-Arg upregulated the expression of iNOS mRNA, and the relative protein levels for iNOS in 200 and 400 μM l-Arg groups were higher than those in 10 and 100 μM l-Arg groups. Collectively, we conclude that the CAT-1 isoform plays a role in l-Arg uptake, and l-Arg-mediated elevation of NO via iNOS promotes the growth of chick intestinal epithelial cells.






Similar content being viewed by others
References
Alberts B, Bray D, Lewis J et al (2002) Molecular biology of the cell, 4th rev. edn. Garland Publishing Inc., New York
Rhoads JM, Wu GY (2009) Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37:111–122
Rhoads JM, Chen W, Gookin J et al (2004) Arginine stimulates intestinal cell migration through a focal adhesion kinase dependent mechanism. Gut 53:514–522
Rhoads JM, Liu YY, Niu XM et al (2008) Arginine stimulates cdx2-transformed intestinal epithelial cell migration via a mechanism requiring both nitric oxide and phosphorylation of p70 S6 kinase. J Nutr 138:1652–1657
Tan B, Yin YL, Kong XF et al (2010) l-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 38:1227–1235
D’Amato JL, Humphrey BD (2010) Dietary arginine levels alter markers of arginine utilization in peripheral blood mononuclear cells and thymocytes in young broiler chicks. Poult Sci 89:938–947
Pance A (2006) Nitric oxide and hormones in breast cancer: allies or enemies? Future Oncol 2:275–288
Wu GY, Morris SM (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Mann GE, Yudilevich DL, Sobrevia L (2003) Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells. Physiol Rev 83:183–252
Closs EI (2002) Expression, regulation and function of carrier proteins for cationic amino acids. Curr Opin Nephrol Hypertens 11:99–107
Verrey F, Closs EI, Wagner CA et al (2004) CATs and HATs: the SLC7 family of amino acid transporters. Pflug Arch Eur J Phy 447:532–542
Baydoun AR, Wileman SM, Wheeler-Jones CP et al (1999) Transmembrane signalling mechanisms regulating expression of cationic amino acid transporters and inducible nitric oxide synthase in rat vascular smooth muscle cells. Biochem J 344:265–272
Simmons WW, Closs EI, Cunningham JM et al (1996) Cytokines and insulin induce cationic amino acid transporter (CAT) expression in cardiac myocytes. Regulation of l-arginine transport and no production by CAT-1, CAT-2A, and CAT-2B. J Biol Chem 271:11694–11702
Vekony N, Wolf S, Boissel JP et al (2001) Human cationic amino acid transporter hCAT-3 is preferentially expressed in peripheral tissues. Biochemistry 40:12387–12394
Wolf S, Janzen A, Vekony N et al (2002) Expression of solute carrier 7A4 (SLC7A4) in the plasma membrane is not sufficient to mediate amino acid transport activity. Biochem J 364:767–775
Cho CH (2001) Current roles of nitric oxide in gastrointestinal disorders. J Physiol Paris 95:253–256
Wallace JL, Miller MJ (2000) Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology 119:512–520
Nathan C, Xie QW (1994) Nitric oxide synthases: roles, tolls, and controls. Cell 78:915–918
Yuan C, He Q, Li JM, Azzam MMM et al (2014) Evaluation of embryonic age and the effects of different proteases on the isolation and primary culture of chicken intestinal epithelial cells in vitro. Anim Sci J (in press)
Kong XF, Tan B, Yin YL et al (2012) l-arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem 23:1178–1183
Nelin LD, Nash HE, Chicoine LG (2001) Cytokine treatment increases arginine metabolism and uptake in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol 281:1232–1239
Zharikov SI, Krotova KY, Belayev L, Block ER (2004) Pertussis toxin activates l-arginine uptake in pulmonary endothelial cells through downregulation of PKC-alpha activity. Am J Physiol Lung Cell Mol Physiol 286:974–983
Schapira RM, Wiessner JH, Morrisey JF et al (1998) l-arginine uptake and metabolism by lung macrophages and neutrophils following intratracheal instillation of silica in vivo. Am J Respir Cell Mol Biol 19:308–315
Humphrey BD, Stephensen BC, Calvert CC, Klasing KC (2006) Lysine deficiency and feed restriction independently alter cationic amino acid transporter expression in chickens (Gallus gallus domesticus). Comp Biochem Physiol A Mol Integr Physiol 143:218–227
Zeng PL, Li XG, Wang XQ et al (2011) The relationship between gene expression of cationic and neutral amino acid transporters in the small intestine of chick embryos and chick breed, development, sex, and egg amino acid concentration. Poult Sci 90:2548–2556
Li YP, Ingmer H, Madsen M, Bang DD (2008) Cytokine responses in primary chicken embryo intestinal cells infected with Campylobacter jejuni strains of human and chicken origin and the expression of bacterial virulence-associated genes. BMC Microbiol 8:107
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using Real-Time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408
Meininger CJ, Wu GY (2002) Regulation of endothelial cell proliferation by nitric oxide. Method Enzymol 352:280–295
Wu GY (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Caso G, Mcnurlan MA, Mcmillan ND et al (2004) Tumour cell growth in culture: dependence on arginine. Clin Sci 107:371–379
Blachier F, Davila AM, Benamouzig R, Tome D (2011) Channelling of arginine in NO and polyamine pathways in colonocytes and consequences. Front Biosci (Landmark Ed) 16:1331–1343
Abdelmagid SA, Too CK (2008) Prolactin and estrogen up-regulate carboxypeptidased to promote nitric oxide production and survival of mcf-7 breast cancer cells. Endocrinology 149:4821–4828
Allen A, Flemstrom G, Graner A, Kivilaakso E (1993) Gastroduodenal mucosal protection. Physiol Rev 73:823–857
Brown JF, Keates AC, Hanson PJ, Whittle BJ (1993) Nitric oxide generators and cGMP stimulate mucus secretion by rat gastric mucosal cells. Am J Physiol Gastrointest Liver Physiol 265:418–422
Lanas A (2008) Role of nitric oxide in the gastrointestinal tract. Arthritis Res Ther. doi:10.1186/ar2465
Nathan C (1997) Inducible nitric oxide synthase: what difference does it make? J Clin Invest 100:2417–2423
Kubes P (1992) Nitric oxide modulates epithelial permeability in the feline small intestine. Am J Physiol Gastrointest Liver Physiol 262:1138–1142
Barry MK, Aloisi JD, Pickering SP, Yeo CJ (1994) Nitric oxide modulates water and electrolyte transport in the ileum. Ann Surg 219:382–388
Hutcheson IR, Whittle BJ, Boughton-Smith NK (1990) Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol 101:815–820
Alican I, Kubes P (1996) A critical role for nitric oxide in intestinal barrier function and dysfunction. Am J Physiol Gastrointest Liver Physiol 270:225–237
Bauchart-Thevret C, Cui LW, Wu GY, Burrin DG (2010) Arginine-induced stimulation of protein synthesis and survival in IPEC-J2 cells is mediated by mTOR but not nitric oxide. Am J Physiol Endocrinol Metab 299:899–909
Strobel J, Mieth M, Endress B et al (2012) Interaction of the cardiovascular risk marker asymmetric dimethylarginine (ADMA) with the human cationic amino acid transporter 1 (CAT1). J Mol Cell Cardiol 53:392–400
Greene B, Pacitti AJ, Souba WW (1993) Characterization of l-arginine transport by pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 264:351–356
Zharikov SI, Block ER (1998) Characterization of l-arginine uptake by plasma membrane vesicles isolated from cultured pulmonary artery endothelial cells. BBA Biomembr 1369:173–183
Hatzoglou M, Fernandez J, Yaman I, Closs E (2004) Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu Rev Nutr 24:377–399
Acknowledgments
This research was supported by the earmarked fund for Modern Argo-Industry Technology Research System of China (No. CARS-41-K17) and the Program for Zhejiang Leading Team of S&T Innovation (No. 2011R50025-04).
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yuan, C., Zhang, X., He, Q. et al. l-arginine stimulates CAT-1-mediated arginine uptake and regulation of inducible nitric oxide synthase for the growth of chick intestinal epithelial cells. Mol Cell Biochem 399, 229–236 (2015). https://doi.org/10.1007/s11010-014-2249-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2249-2