Abstract
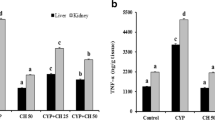
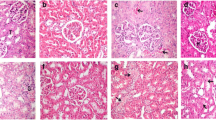
Cyclophosphamide (CPM), an alkylating agent is used as an immunosuppressant in rheumatoid arthritis and in the treatment of several cancers as well. In this study, Ellagic acid (EA), a naturally occurring plant polyphenol, was evaluated for its antigenotoxicity and antioxidant efficacy against the CPM-induced renal oxidative stress and genotoxicity in Swiss albino mice. The mice were given a prophylactic treatment of EA orally at a dose of 50 and 100 mg/kg body weight (b wt) for seven consecutive days before the administration of a single intraperitoneal (i.p.) injection of CPM at 50 mg/kg b wt. The modulatory effects of EA on CPM-induced nephrotoxicity and genotoxicity were investigated by assaying oxidative stress biomarkers, serum kidney toxicity markers, DNA fragmentation, alkaline unwinding assay, micronuclei (MN) assay, and by histopathological examination of kidney tissue. A single intraperitoneal administration of CPM in mice increased malondialdehyde level with depletion in glutathione content, antioxidant enzymes activities, viz. glutathione peroxidase, glutathione reductase, catalase, quinone reductase, induced DNA strand breaks, and MN induction. EA oral administration at both doses caused significant reduction in their levels, restoration in the activities of antioxidant enzymes, reduction in MN formation, and DNA fragmentation. Serum toxicity marker enzymes like BUN, creatinine, and LDH were also increased after CPM treatment which was significantly decreased in EA pretreated groups. Present findings suggest a prominent role of EA against CPM-induced renal injury, DNA damage, and genotoxicity.





Similar content being viewed by others
Abbreviations
- EA:
-
Ellagic acid
- CPM:
-
Cyclophosphamide
- GSH:
-
Reduced glutathione
- GPx:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- XO:
-
Xanthine oxidase
- MDA:
-
Malondialdehyde
- BUN:
-
Blood urea nitrogen
- LDH:
-
Lactate dehydrogenase
References
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477
Morton LW, Abu-Amsha Caccetta R, Puddey IB, Croft KD (2000) Chemistry and biological effects of dietary phenolic compounds: relevance to cardiovascular disease. Clin Exp Pharmacol Physiol 27:152–159
Zhao Z, Egashira Y, Sanada H (2004) Ferulic acid is quickly absorbed from rat stomach as the free form and then conjugated mainly in liver. J Nutr 134:3083–3088
Surh YJ (2003) Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3:768–780
Ippoushi K, Takeuchi A, Azuma K (2009) Prevention of peroxynitrite-induced oxidation and nitration reactions by ellagic acid. Food Chem 112:185–188
Hannum SM (2004) Potential impact of strawberries on human health: a review of the science. Crit Rev Food Sci Nutr 44:1–17
Heur YH, Zeng W, Stoner GD, Nemeth GA, Hilton B (1992) Synthesis of ellagic acid O-alkyl derivatives and isolation of ellagic acid as a tetrahexanoyl derivative from Fragaria ananassa. J Nat Prod 55:1402–1407
Mandal S, Ahuja A, Shivapurkar NM, Cheng SJ, Groopman JD, Stoner GD (1987) Inhibition of aflatoxin B1 mutagenesis in Salmonella typhimurium and DNA damage in cultured rat and human tracheobronchial tissues by ellagic acid. Carcinogenesis 8:1651–1656
Papoutsi Z, Kassi E, Tsiapara A, Fokialakis N, Chrousos GP, Moutsatsou P (2005) Evaluation of estrogenic/antiestrogenic activity of ellagic acid via the estrogen receptor subtypes ERα and ERβ. J Agric Food Chem 53:7715–7720
Yuce A, Ateşşahin A, Ceribası AO, Aksakal M (2007) Ellagic acid prevents cisplatin induced oxidative stress in liver and heart tissue of rats. Basic Clin Pharmacol Toxicol 101:345–349
Turk G, Atessahin A, Sonmez M, Ceribas AO, Yuce A (2008) Improvement of cisplatin induced injuries to sperm quality, the oxidant–antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil Steril 89:1474–1481
Yu Y-M, Chang W-C, Wu C-H, Chiang S-Y (2005) Reduction of oxidative stress and apoptosis in hyperlipidemic rabbits by ellagic acid. J Nutr Biochem 16:675–681
Atessahin A, Ceribaş AO, Yuce A, Bulmus O, Ckim G (2007) Role of ellagic acid against cisplatin-induced nephrotoxicity and oxidative stress in rats. Basic Clin Pharmacol Toxicol 100:121–126
Hwang JM, Cho JS, Kim TH, Lee YI (2010) Ellagic acid protects hepatocytes from damage by inhibiting mitochondrial production of reactive oxygen species. Biomed Pharmacother 64:264–270
Rogerio AP, Fontanari C, Borducchi E, Keller AC, Rusco M, Soares EG, Albuquerque DA, Faccioli LH (2008) Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma. Eur J Pharmacol 580:262–270
de Boer JG, Yang H, Holcroft J, Skov K (2004) Chemoprotection against N-nitrosomethylbenzylamine-induced mutation in the rat esophagus. Nutr Cancer 50:168–173
Ahn D, Putt D, Kresty L, Stoner GD, Fromm D, Hollenberg PF (1996) The effects of dietary ellagic acid on rat hepatic and esophageal mucosal cytochromes P450 and phase II enzymes. Carcinogenesis 17:821–828
Shepherd AG, Manson MM, Ball HW, McLellan LI (2000) Regulation of rat glutamate-cysteine ligase (γ-glutamylcysteine synthetase) subunits by chemopreventive agents and in aflatoxin B1-induced preneoplasia. Carcinogenesis 21:1827–1834
van der Logt EM, Roelofs HM, Nagengast FM, Peters WH (2003) Induction of rat hepatic and intestinal UDP-glucuronosyltransferases by naturally occurring dietary anticarcinogens. Carcinogenesis 24:1651–1656
Baumann F, Preiss R (1973) Cyclophosphamide and related anticancer drugs. J Chromatogr B 764:173–192
Fleming RE (1997) An overview of cyclophosphamide and ifosfamide pharmacology. Pharmacotherapy 17:1465–1545
Perini P, Calabrese M, Rinaldi L, Gallo P (2007) The safety profile of cyclophosphamide in multiple sclerosis therapy. Expert Opin Drug Saf 6:183–190
Uber WE, Self SE, Van Bakel AB, Pereira NL (2007) Acute antibody-mediated rejection following heart transplantation. Am J Transplant 7:2064–2074
IARC (1987) IARC monograph on the evaluation of carcinogenicity: an update of IARC monographs 1 to 42. International Agency for Research on Cancer Supplement 7
Hales BE (1982) Comparison of the mutagenicity and teratogenicity of cyclophosphamide and its active metabolites, 4-hydroxycyclophosphamide, phosphoramide mustard and acrolein. Cancer Res 42:3016–3021
Patel JM (1987) Stimulation of cyclophosphamide-induced pulmonary microsomal lipid peroxidation by oxygen. Toxicology 45:79–91
Tripathi DN, Jena GB (2008) Astaxanthin inhibits cytotoxic and genotoxic effects of cyclophosphamide in mice germ cells. Toxicology 27:96–103
Travis LB, Curtis RE, Glimelius B, Holowaty EJ, Van Leeuwen FE, Lynch CF, Hagenbeek A, Stovall M, Banks PM, Adami J et al (1995) Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin’s lymphoma. J Natl Cancer Inst 87:524–530
Sladek N (1971) Metabolism of cyclophosphamide by rat hepatic microsomes. Cancer Res 1:901–908
Sladek NE (1988) Metabolism of oxazaphosphorines. Pharmacol Ther 37:301–355
Roy P, Yu LJ, Crespi CL, Waxman DJ (1999) Development of a substrate-activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P-450 profiles. Drug Metab Dispos 27:655–666
Bagley C, Bostick F (2001) Clinical pharmacology of cyclophosphamide. Cancer Res 33:226–235
Lin SS, Hung CF, Ho CC, Liu YH, Ho HC, Chung JG (2000) Effects of Ellagic acid by oral administration on N-acetylation and metabolism of 2-aminofluorene in rat brain tissues. Neurochem Res 25(11):1503–1508
Suzuki N, Masamune A, Kikuta K, Watanabe T, Satoh K, Shimosegawa T (2009) Ellagic acid inhibits pancreatic fibrosis in male Wistar Bonn/Kobori rats. Dig Dis Sci 54(4):802–810
Levy L, Harris R (1977) Effect of N-acetyl cysteine on some aspects of cyclophosphamide induced toxicity and immunesupression. Biochem Pharmacol 26(11):1015–1020
Chakraborty P, Sk UH, Murmu N, Das JK, Pal S, Bhattacharya S (2009) Modulation of cyclophosphamide-induced cellular toxicity by diphenylmethyl selenocyanate in vivo, an enzymatic study. J Cancer Mol 4(6):183–189
Nafees S, Tanveer S, Wani A, Rashid S, Ali N and Sultana S (2011) Modulatory effects of gentisic acid against genotoxicity and hepatotoxicity induced by cyclophosphamide in Swiss albino mice. J Pharm Pharmacol 64(2):259–267
Schmid W (1975) The micronucleus test. Mutat Res 31:9–15
Gollapudi B, McFadden LG (1995) Sample size for the estimation of polychromatic to normochromatic erythrocyte ratio in the bone marrow micronucleus test. Mutat Res 347:97–99
Tahir M, Sultana S (2011) Chrysin modulates ethanol metabolism in Wistar rats: a promising role against organ toxicities. Alcohol Alcohol 46:383–392
Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR (1974) Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4-bromobezene oxide as the hepatotoxic metabolite. Pharmacology 11:151
Carlberg I, Mannervik B (1985) Glutathione level in rat brain. J Biol Chem 250:4480–4575
Mohandas M, Marshall JJ, Duggin GG, Horvath JS, Tiller D (1984) Differential distribution of glutathione and glutathione related enzymes in rabbit kidney. Cancer Res 44:5086–5091
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Wright JR, Colby HD, Miles PR (1981) Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys 206:296–304
Stripe F, Della Corte E (1969) The regulation of rat liver xanthine oxidase. J Biol Chem 244:3855–3863
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods in oxygen radical research. CRC Press, Boca Raton, pp 283–284
Kornberg A (1955) Lactic dehydrogenase of muscle. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol I. Academic Press, New York, pp 441–443
Lowry OH, Rosebrough NJ, Farr A, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Hengstler JG, Hengst A, Fuchs J, Tanner B, Pohl J, Oesch F (1997) Induction of DNA cross links and DNA strand lesions by cyclophosphamide after inactivation by cytochrome P-450 2B1. Mutat Res 373:215–223
Haque R, Bin-Hafeez B, Parvez S, Pandey S, Sayeed I, Ali M, Raisuddin S (2003) Aqueous extract of walnut (Juglans regia L.) protects mice against cyclophosphamide-induced biochemical toxicity. Hum Exp Toxicol 22:473–480
Selvakumar E, Prahalathan C, Mythili Y, Varalakshmi P (2005) Mitigation of oxidative stress in cyclophosphamide-challenged hepatic tissue by dl-alpha-lipoic acid. Mol Cell Biochem 272:179–185
Cooper JA, Merrill WW, Reynolds HY (1986) Cyclophosphamide modulation of bronchoalveolar cellular populations and macrophage oxidative metabolism: possible mechanisms of pulmonary pharmacotoxicity. Am Rev Respir Dis 134:108–114
Kehrer JP, Biswal SS (2000) The molecular effects of acrolein. Toxicol Sci 57:6–15
Tripathi DN, Jena GB (2009) Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: a study in mice. Chem Biol Interact 180:398–406
Bhatia K, Kaur M, Atif F, Ali M, Rehman H, Rahman S, Raisuddin S (2006) Aqueous extract of Trigonella foenum-graecum L. ameliorates additive urotoxicity of buthionine sulfoximine and cyclophosphamide in mice. Food Chem Toxicol 44:1744–1750
Cagler K, Kinalp C, Arpaci F, Turan M, Saglam K, Ozturk B, Komurcu S, Yavuz, Yenicesu M, Ozet A, Vural A (2002) Cumulative prior dose of cisplatin as a cause of the nephrotoxicity of high-dose chemotherapy followed by autologous stem-cell transplantation. Nephrol Dial Transplant 17:1931–1935
Abrahama P, Indiranib K, Sugumar E (2007) Effect of cyclophosphamide treatment on selected lysosomal enzymes in the kidney of rats. Exp Toxicol Pathol 59:143–149
Salamone MF, Heddle JA (1983) The bone marrow micronucleus assay: rationale for a revised protocol. In: de Serres FJ (ed) Chemical mutagens: principles and methods for their detection, vol 8. Plenum, New York, pp 111–149
Al-Majed AA, Al-Yahya AA, Al-Bekairi AM, Al-Shabanah OA, Qureshi S (2006) Studies on the cytological and biochemical effects of valerian in somatic and germ cells of Swiss albino mice. Food Chem Toxicol 44:1830–1837
Acknowledgment
The authors are thankful to CCRUM (AYUSH), Ministry of Health and Family Welfare, Govt. of India for providing funds to carry out this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rehman, M.U., Tahir, M., Ali, F. et al. Cyclophosphamide-induced nephrotoxicity, genotoxicity, and damage in kidney genomic DNA of Swiss albino mice: the protective effect of Ellagic acid. Mol Cell Biochem 365, 119–127 (2012). https://doi.org/10.1007/s11010-012-1250-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-012-1250-x