Abstract
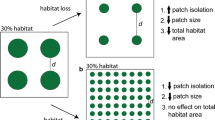
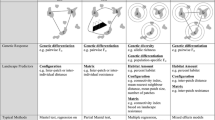
A common approach used to estimate landscape resistance involves comparing correlations of ecological and genetic distances calculated among individuals of a species. However, the location of sampled individuals may contain some degree of spatial uncertainty due to the natural variation of animals moving through their home range or measurement error in plant or animal locations. In this study, we evaluate the ways that spatial uncertainty, landscape characteristics, and genetic stochasticity interact to influence the strength and variability of conclusions about landscape-genetics relationships. We used a neutral landscape model to generate 45 landscapes composed of habitat and non-habitat, varying in percent habitat, aggregation, and structural connectivity (patch cohesion). We created true and alternate locations for 500 individuals, calculated ecological distances (least-cost paths), and simulated genetic distances among individuals. We compared correlations between ecological distances for true and alternate locations. We then simulated genotypes at 15 neutral loci and investigated whether the same influences could be detected in simple Mantel tests and while controlling for the effects of isolation-by-distance using the partial Mantel test. Spatial uncertainty interacted with the percentage of habitat in the landscape, but led to only small reductions in correlations. Furthermore, the strongest correlations occurred with low percent habitat, high aggregation, and low to intermediate levels of cohesion. Overall genetic stochasticity was relatively low and was influenced by landscape characteristics.





Similar content being viewed by others
References
Akaike H (1973) Information theory as an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (eds) Second international symposium on information theory. Akademiai Kiado, Budapest, pp 267–281
Anderson DR, Burnham KP, Thompson WL (2000) Null hypothesis testing: problems, prevalence, and an alternative. J Wildl Manag 64(4):912–923
Balkenhol N, Gugerli F, Cushman SA, Waits LP, Coulon A, Arntzen JW, Holderegger R, Wagner H (2009) Identifying future research needs in landscape genetics: where to from here? Landscape Ecol 24:455–463
Bowcock AM, Ruiz-Linares A, Tomfohrde J, Minch E, Kidd JR, Cavalli-Sforza LL (1994) High resolution of human evolutionary trees with polymorphic micorsatellites. Nature 368:455–457
Braunisch V, Hirzel A, Segelbacher G (2010) Modelling functional landscape connectivity from genetic population structure: a new spatially explicit approach. Mol Ecol 19:3664–3678
Bruggeman DJ, Wiegand T, Fernandez N (2010) The relative effects of habitat loss and fragmentation on population genetic structure. Mol Ecol 19:3691–3697
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Cushman SA, Landguth EL (2010) Spurious correlations and inference in landscape genetics. Mol Ecol 19:3592–3602
Cushman SA, McKelvey KS, Hayden J, Schwartz MK (2006) Gene flow in complex landscapes: testing multiple hypotheses with causal modeling. American Naturalist 168:486–499
Cushman SA, Shirk A, Landguth EL (2012) Separating the effects of habitat area, fragmentation and matrix resistance on genetic differentiation in complex landscapes. Landscape Ecol. doi:10.1007/s10980-011-9693-0
Dixo M, Metzger JP, Morgante JS, Zamudio KR (2009) Habitat fragmentation reduces genetic diversity and connectivity among toad populations in the Brazilian Atlantic Coastal Forest. Biol Conserv 142:1560–1569
Epperson BK, McRae B, Scribner K, Cushman SA, Rosenberg MS, Fortin M-J, James PMA, Murphy M, Manel S, Legendre P, Dale MRT (2010) Utility of computer simulations in landscape genetics. Mol Ecol 19:3540–3564
Epps CW, Palsboll PJ, Wehausen JD, Roderck GK, Ramey RR II, McCullough DR (2005) Highways block gene flow and cause a rapid decline in genetic diversity of desert bighorn sheep. Ecol Lett 8:1029–1038
Epps CW, Wehausen JD, Bleich VC, Torres SG, Brashares JS (2007) Optimizing dispersal and corridor models using landscape genetics. J Appl Ecol 44:714–724
ESRI (1999–2008) Environmental System Research Institute, Redlands
Ficetola GF, Garner TWJ, Biernard FD (2007) Genetic diversity, but not hatching success, is jointly affected by postglacial colonization and isolation in the threatened frog, Rana latastei. Mol Ecol 9:1787–1797
Fortin M-J, Boots B, Csillag F, Remmel TK (2003) On the role of spatial stochastic models in understanding landscape indices in ecology. Oikos 102:203–212
Gardner RH (1999) QRULE: a program for the generation of random maps and the analysis of spatial patterns. In: Klopatek JM, Gardner RH (eds) Landscape ecological analysis: issues and applications. Springer-Verlag, New York, pp 280–303
Goslee SC, Urban DL (2007) The ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw 22:1–19
Holderegger R, Wagner HH (2008) Landscape genetics. Bioscience 3:199–207
Jacquemyn H, Honnay O, Galbusera P, Roldan-Ruiz I (2004) Genetic structure of the forest herb Primula elatior in a changing landscape. Mol Ecol 13:211–219
Krofel M, Filacorda A, Jerina K (2010) Mating-related movements of male brown bears on the periphery of an expanding population. Ursus 21:23–29
Landguth EL, Cushman SA (2010) CDPOP: an individual-based, cost-distance spatial population genetics model. Mol Ecol Resour 10:156–161
Landguth EL, Cushman SA, Schwartz MK, McKelvey KS, Murphy M, Luikarts G (2010) Quantifying the lag time to detect barriers in landscape genetics. Mol Ecol 19:4179–4191
Legendre P, Fortin M-J (2010) Comparison of the Mantel test and alternative approaches for detecting complex multivariate relationships in the spatial analysis of genetic data. Mol Ecol Resour 10:831–844
Lovari S, Barolommei P, Meschi F, Pezzo F (2008) Going out to mate: excursion behaviour of female roe deer. Ethology 114:886–896
Manel S, Schwartz MK, Luikart G, Taberlet P (2003) Landscape genetics: combining landscape ecology and population genetics. Trends Ecol Evol 18:189–197
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
McGarigal K, Cushman SA, Neel MC, Ene E (2002) FRAGSTATS: spatial pattern analysis program for categorical maps. Computer software program produced by the authors at the University of Massachusetts, Amherst
McRae BH (2006) Isolation by resistance. Evolution 60:1551–1561
McRae BH, Beier P, Dewald LE, Huynh LY, Keim P (2005) Habitat barriers limit gene flow and illuminate historical events in a wide-ranging carnivore, the American puma. Mol Ecol 14:1965–1977
Murphy MA, Evans JS, Cushman SA, Storfer A (2008) Representing genetic variation as continuous surfaces: an approach for identifying spatial dependency in landscape genetic studies. Ecography 31:685–697
Murphy MA, Evans JS, Storfer A (2010) Quantifying Bufo boreas connectivity in Yellowstone National Park with landscape genetics. Ecology 91:261–262
Neel MC, Cushman SA, McGarigal K (2004) Behavior and stability of landscape metrics across controlled gradients of landscape structure. Landscape Ecol 19:435–455
Pinheiro J, Bates D, DebRoy S, Sarkar D, The R Development Core Team (2011) Nlme: linear and nonlinear mixed effects models. R package version 3:1–100
Pinto N, Keitt TH (2009) Beyond the least-cost path: evaluating corridor redundancy with a graph theoretic approach. Landscape Ecol 24:253–266
R Development Core Team (2010). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Rayfield B, Fall A, Fortin M-J (2010) The sensitivity of least-cost habitat graphs to relative cost surface values. Landscape Ecol 25:519–532
Richard E, Morellet N, Cargnelutti B, Angibault JM, Vanpe C, Hewison AJM (2008) Ranging behaviour and excursions of female roe deer during the rut. Behav Process 79:28–35
Schumaker NH (1996) Using landscape indices to predict habitat connectivity. Ecology 77:1210–1225
Schwartz MK, Copeland JP, Anderson NJ, Squires JR, Inman RM, McKelvey KS, Pilgrim KL, Waits LP, Cushman SA (2009) Wolverine gene flow across a narrow climatic niche. Ecology 90:3222–3232
Segelbacher G, Cushman SA, Epperson BK, Fortin M-J, Francois O, Hardy DJ, Holderegger R, Taberlet P, Waits LP, Manel S (2010) Applications of landscape genetics in conservation biology: concepts and challenges. Conserv Genet 11:375–385
Shirk AJ, Wallin DO, Cushman SA, Rice CG, Wahrheit KA (2010) Inferring landscape effects on gene flow: a new model selection framework. Mol Ecol 19:3603–3619
Short Bull RA, Cushman SA, Mace R, Chilton T, Kendall KC, Landguth EL, Schwartz MK, McKelvey KS, Allendorf FW, Luikart G (2011) Why replication is important in landscape genetics: American black bear in the Rocky Mountains. Mol Ecol 20:1092–1107
Smouse PE, Long JC, Sokal RR (1986) Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Syst Zool 35:627–632
Sork VL, Smouse PE (2004) Genetic analysis of landscape connectivity in tree populations. Landscape Ecol 21:821–836
Spear SF, Balkenhol N, Fortin M-J, Mcrae BH, Scribner K (2010) Use of resistance surfaces for landscape genetic studies: considerations for parameterization and analysis. Mol Ecol 19:3576–3591
Storfer A, Murphy M, Evans JS, Goldberg CS, Robinson S, Spear SF, Dezzani R, Delmelle E, Vierling L, Waits LP (2007) Putting the “landscape” in landscape genetics. Heredity 98:128–142
Storfer A, Murphy M, Spear S, Holderegger R, Waits L (2010) Landscape genetics: where are we now? Mol Ecol 19:3496–3514
Wasserman TN, Cushman SA, Schwartz MK, Wallin DO (2010) Spatial scaling and model inference in landscape genetics: Martes Americana in northern Idaho. Landscape Ecol 25:1601–1612
Wasserman TN, Cushman SA, Shirk AS, Landguth EL, Littell JS (2011) Simulating the effects of climate change on population connectivity of American marten (Martes Americana) in the northern rocky Mountains, USA. Landscape Ecol. doi:10.1007/s10980-011-9653-8)
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We thank Brent Burch, Kevin McGarigal, and John Citta for useful discussions on modeling random effects. This study resulted from a distributed graduate seminar (developing best practices for testing landscape effects on gene flow), conducted through the National Center for Ecological Analysis and Synthesis (NCEAS), a center funded by the National Science Foundation Grant #EF-0553768, the University of California, Santa Barbara, and the State of California. We thank Carisa Stansbury and Rodrigo Cisneros for assistance with preliminary simulations. This was a truly collaborative project. Contributions of each coauthor are listed in Supplement II. We appreciate our individual sources of support, including scholarship, fellowship and research assistantship providers. We also thank 2 anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Graves, T.A., Wasserman, T.N., Ribeiro, M.C. et al. The influence of landscape characteristics and home-range size on the quantification of landscape-genetics relationships. Landscape Ecol 27, 253–266 (2012). https://doi.org/10.1007/s10980-011-9701-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-011-9701-4