Abstract
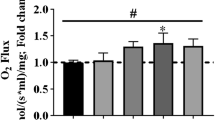
The present study investigated changes in autolysis of three calpain isoforms in skeletal muscles undergoing eccentric contractions (ECC), leading to prolonged force deficits. Rat extensor digitorum longus and tibialis anterior muscles were exposed to 200-repeated ECC in situ, excised immediately after or 3 or 6 days after cessation of ECC, and used for measures of force output and for biochemical analyses. Full restoration of tetanic force in ECC-treated muscles was not attained until 6 days of recovery. Maximal calpain activity determined by a fluorogenic substrate was unaltered immediately after ECC, but increased to 313 and 450 % after 3 and 6 days, respectively. Increases in the amount of autolyzed calpain-3 were apparent immediately and developed progressively with recovery time, whereas elevations of autolyzed μ- and m-calpain occurred after 3 and 6 days, respectively. The protein content was augmented only in m-calpain. It is suggested that the three calpain isoforms may be involved in the dismantling, repair, remodeling and/or regeneration processes in ECC-treated muscles.






Similar content being viewed by others
References
Allen DG, Whitehead NP, Yeung EW (2005) Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: role of ionic changes. J Physiol 567(3):723–735
Balcerzak D, Poussard S, Brustis JJ, Elamrani N, Soriano M, Cottin P, Ducastaing A (1995) An antisense oligodeoxyribonucleotide to m-calpain mRNA inhibits myoblast fusion. J Cell Sci 108(5):2077–2082
Belcastro AN, Shewchuk LD, Raj DA (1998) Exercise-induced muscle injury: a calpain hypothesis. Mol Cel Biochem 179(1–2):135–145
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Corona BT, Balog EM, Doyle JA, Rupp JC, Luke RC, Ingalls CP (2010) Junctophilin damage contributes to early strength deficits and EC coupling failure after eccentric contractions. Am J Physiol 298(2):C365–C376
Diaz BG, Moldoveanu T, Kuiper MJ, Campbell RL, Davies PL (2004) Insertion sequence 1 of muscle-specific calpain, p94, acts as an internal propeptide. J Biol Chem 279(26):27656–27666
Douillard A, Galbes O, Rossano B, Vernus B, Bonnieu A, Candau R, Py G (2011) Time course in calpain activity and autolysis in slow and fast skeletal muscle during clenbuterol treatment. Can J Physiol Pharmacol 89(2):117–125
Faulkner JA, Jones DA, Round JM (1989) Injury to skeletal muscles of mice by forced lengthening during contractions. Q J Exp Physiol 74(5):661–670
Féasson L, Stockholm D, Freyssenet D, Richard I, Duguez S, Beckmann JS, Denis C (2002) Molecular adaptations of neuromuscular disease-associated proteins in response to eccentric exercise in human skeletal muscle. J Physiol 543(1):297–306
Gilchrist JS, Wang KK, Katz S, Belcastro AN (1992) Calcium-activated neutral protease effects upon skeletal muscle sarcoplasmic reticulum protein structure and calcium release. J Biol Chem 267(29):20857–20865
Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiol Rev 83(3):731–801
Gollnick PD, Timson BF, Moore RL, Riedy M (1981) Muscular enlargement and number of fibers in skeletal muscle. J Appl Physiol 50(5):936–943
Ingalls CP, Warren GL, Armstrong RB (1998) Dissociation of force production from MHC and actin contents in muscles injured by eccentric contractions. J Muscle Res Cell Motil 19(3):215–224
Ingalls CP, Warren GL, Zhang J-Z, Hamilton SL, Armstrong RB (2004) Dihydropyridine and ryanodine receptor binding after eccentric contraction. J Appl Physiol 96(3):1619–1625
Kanzaki K, Kuratani M, Matsunaga S, Mishima T, Usui S, Wada M (2010a) Effect of eccentric contractions on in vitro Na+-K+-ATPase activity and sarcoplsmic reticulum Ca2+-sequestering in rat skeletal muscle. Jpn J Phys Fitness Sports Med 59(4):337–348 (in Japanese)
Kanzaki K, Kuratani M, Mishima T, Matsunaga S, Yanaka N, Usui S, Wada M (2010b) The effects of eccentric contraction on myofibrillar proteins in rat skeletal muscle. Eur J Appl Physiol 110(5):943–952
Kisselev AF, Goldberg AL (2005) Monitoring activity and inhibition of 26S proteasomes with fluorogenic peptide substrates. Methods Enzymol 398:364–378
Lehti M, Kivelä R, Komi P, Komulainen J, Kainulainen H, Kyröläinen H (2009) Effects of fatiguing jumping exercise on mRNA expression of titin-complex proteins and calpains. J Appl Physiol 106(4):1419–1424
Lynch GS, Fary CJ, Williams DA (1997) Quantitative measurement of resting skeletal muscle [Ca2+]i following acute and long-term downhill running exercise in mice. Cell Calcium 22(5):373–383
Murphy RM (2010) Calpains, skeletal muscle function and exercise. Clin Exp Pharmacol Physiol 37(3):385–391
Murphy RM, Lamb GD (2009) Endogenous calpain-3 activation is primarily governed by small increases in resting cytoplasmic [Ca2+] and is not dependent on stretch. J Biol Chem 284(12):7811–7819
Murphy RM, Snow RJ, Lamb GD (2006a) μ-Calpain and calpain-3 are not autolyzed with exhaustive exercise in humans. Am J Physiol 290(1):C116–C122
Murphy RM, Verburg E, Lamb GD (2006b) Ca2+ activation of diffusible and bound pools of u-calpain in rat skeletal muscle. J Physiol 576(2):595–612
Murphy RM, Goodman CA, McKenna MJ, Bennie J, Leikis M, Lamb GD (2007) Calpain-3 is autolyzed and hence activated in human skeletal muscle 24 h following a single bout of eccentric exercise. J Appl Physiol 103(3):931–936
Murphy RM, Vissing K, Latchman H, Lamboley C, McKenna MJ, Overgaard K, Lamb GD (2011) Activation of skeletal muscle calpain-3 by eccentric exercise in humans does not result in its translocation to the nucleus or cytosol. J Appl Physiol 111(5):1448–1458
Murphy RM, Dutka TL, Horvath D, Bell JR, Delbridge LM, Lamb GD (2013) Ca2+-dependent proteolysis of junctophilin-1 and junctophilin-2 in skeletal and cardiac muscle. J Physiol 591(3):719–729
Ojima K, Kawabata Y, Nakao H, Nakao K, Doi N, Kitamura F, Ono Y, Hata S, Suzuki H, Kawahara H, Bogomolovas J, Witt C, Ottenheijm C, Labeit S, Granzier H, Toyama-Sorimachi N, Sorimachi M, Suzuki K, Maeda T, Abe K, Aiba A, Sorimachi H (2010) Dynamic distribution of muscle-specific calpain in mice has a key role in physical-stress adaptation and is impaired in muscular dystrophy. J Clin Invest 120(8):2672–2683
Pette D, Staron RS (1990) Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol 116:1–76
Raser KJ, Posner A, Wang KKW (1995) Casein zymography: a method to study micro-calpain, m-calpain, and their inhibitory agents. Arch Biochem Biophys 319(1):211–216
Rathbone CR, Wenke JC, Warren GL, Armstrong RB (2003) Importance of satellite cells in the strength recovery after eccentric contraction-induced muscle injury. Am J Physiol 285(6):R1490–R1495
Shevchenko S, Feng W, Varsanyi M, Shoshan-Barmatz V (1998) Identification, characterization and partial purification of a thiol-protease which cleaves specifically the skeletal muscle ryanodine receptor/Ca2+ release channel. J Membr Biol 161(1):33–43
Solomon V, Goldberg AL (1996) Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 271(43):26690–26697
Sultan KR, Dittrich BT, Pette D (2000) Calpain activity in fast, slow, transforming and regenerating skeletal muscle. Am J Physiol 279(3):C639–C647
Taveau M, Bourg N, Sillon G, Roudaut C, Bartoli M, Richard I (2003) Calpain 3 is activated through autolysis within the active site and lyses sarcomeric and sarcolemmal components. Mol Cell Biol 23(24):9127–9135
Tidball JG, Spencer MJ (2002) Expression of a calpastatin transgene slows muscle wasting and obviates changes in myosin isoform expression during murine muscle disuse. J Physiol 545(3):819–828
Verburg E, Murphy RM, Richard I, Lamb GD (2009) Involvement of calpains in Ca2+-induced disruption of excitation-contraction coupling in mammalian skeletal muscle fibers. Am J Physiol 296(5):C1115–C1122
Wada M, Kuratani M, Kanzaki K (2013) Calcium kinetics of saercoplasmic reticulum and muscle fatigue. J Phys Fitness Sports Med 2(2):169–178
Zhang BT, Yeung SS, Allen DG, Qin L, Yeung EW (2008) Role of the calcium-calpain pathway in cytoskeletal damage after eccentric contractions. J Appl Physiol 105(1):352–357
Zhang BT, Whitehead NP, Gervasio OL, Reardon TF, Vale M, Fatkin D, Dietrich A, Yeung EW, Allen DG (2012) Pathways of Ca2+ entry and cytoskeletal damage following eccentric contractions in mouse skeletal muscle. J Appl Physiol 112(12):2077–2086
Acknowledgments
Financial support for this study was received from Grants-Aid for Scientific Research of Japan (No. 24500788; Wada).
Conflict of interest
We do not have any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanzaki, K., Kuratani, M., Matsunaga, S. et al. Three calpain isoforms are autolyzed in rat fast-twitch muscle after eccentric contractions. J Muscle Res Cell Motil 35, 179–189 (2014). https://doi.org/10.1007/s10974-014-9378-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-014-9378-9