Abstract
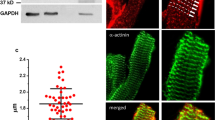
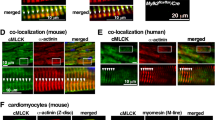
Spinophilin (SPN) is a ubiquitously expressed scaffolding protein that interacts through several binding modules with a variety of target proteins. Thus, SPN bundles F-actin, targets protein phosphatase 1 to the ryanodine receptor, and targets regulators of G-protein signaling to G-protein coupled receptors in cardiomyocytes. In this work we studied the role of SPN on cardiomyocyte morphology, function, and β-adrenergic responsiveness using a homozygous SPN knock-out mouse model (SPN−/−). We show that spinophilin deficiency significantly (1) reduced cardiomyocyte length, (2) increases both Ca2+ amplitude and maximal rate of Ca2+ rise during systole, and (3) decreased shortening amplitude and maximal rate of shortening, while (4) β-adrenergic stimulation remained intact. Our data suggest that spinophilin is an upstream regulator required for normal growth and excitation–contraction coupling, but is dispensable for β-adrenergic stimulation of adult cardiomyocytes.

Similar content being viewed by others
References
Allen PB, Ouimet CC, Greengard P (1997) Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci USA 94:9956–9961
Berndt N (1999) Protein dephosphorylation and the intracellular control of the cell number. Front Biosci 4:22–42
Bers DM (2004) Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol 37:417–429
da Costa-Goncalves AC, Tank J, Plehm R, Diedrich A, Todiras M, Gollasch M, Heuser A, Wellner M, Bader M, Jordan J, Luft FC, Gross V (2008) Role of the multidomain protein spinophilin in blood pressure and cardiac function regulation. Hypertension 52:702–707
Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P (2000) Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci USA 97:9287–9292
Fernandez A, Brautigan DL, Mumby M, Lamb NJ (1990) Protein phosphatase type-1, not type-2A, modulates actin microfilament integrity and myosin light chain phosphorylation in living nonmuscle cells. J Cell Biol 111:103–112
Ferrer I, Blanco-Aparicio C, Peregrina S, Cañamero M, Fominaya J, Cecilia Y, Lleonart M, Hernandez-Losa J, Ramon Y Cajal S, Carnero A (2011) Spinophilin acts as a tumor suppressor by regulating Rb phosphorylation. Cell Cycle 15:10 [Epub ahead of print]
Kim SS, Wang H, Li XY, Chen T, Mercaldo V, Descalzi G, Wu LJ, Zhuo M (2011) Neurabin in the anterior cingulate cortex regulates anxiety-like behavior in adult mice. Mol Brain [Epub ahead of print]
Liu CW, Wang RH, Dohadwala M, Schonthal AH, Villa-Moruzzi E, Berndt N (1999) Inhibitory phosphorylation of PP1alpha catalytic subunit during the G(1)/S transition. J Biol Chem 274:29470–29475
Lu R, Chen Y, Cottingham C, Peng N, Jiao K, Limbird LE, Wyss JM, Wang Q (2010) Enhanced hypotensive, bradycardic, and hypnotic responses to a2-adrenergic agonists in spinophilin-null mice are accompanied by increased G protein coupling to the a2A-adrenergic receptor. Mol Pharmacol 78:279–286
Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, Rosemblit N, Marks AR (2001) Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol 153:699–708
Ragusa M, Dancheck B, Critton DA, Nairn AC, Page R, Peti W (2010) Spinophilin directs protein phosphatase 1 specificity by blocking substrate binding sites. Nat Struc Mol Biol 17:459–466
Sarrouilhe D, di Tommaso A, Metaye T, Ladeveze V (2006) Spinophilin: from partners to functions. Biochimie 88:1099–1113
Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Mizoguchi A, Takai Y (1998) Neurabin-II/spinophilin. An actin filament-binding protein with one pdz domain localized at cadherin-based cell–cell adhesion sites. J Biol Chem 273:3470–3475
Trafford AW, D.úaz ME, Sibbring GC, Eisner DA (2000) Modulation of CICR has no maintained effect on systolic Ca2+ simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J Physiol 522:259–270
Verduyn SC, Zaremba R, von der Velden J, Stienen GJ (2007) Effects of contractile protein phosphorylation on force development in permeabilized rat cardiac myocytes. Basic Res Cardiol 102:476–487
Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, Barnes AP, Milgram SL, Penninger JM, Allen PB, Greengard P, Muallem S (2005) Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol 7:405–411
Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P (1999) Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci 2:13–17
Zhao S, Brandt NR, Caswell AH, Lee EY (1998) Binding of the catalytic subunit of protein phosphatase-1 to the ryanodine-sensitive calcium release channel protein. Biochemistry 37:18102–18109
Acknowledgments
We thank Petra Sakel (MDC, Berlin, Germany) for technical assistance and for preparing the primary adult cardiomyocytes. We thank Wolfgang-Peter Schlegel for technical support of the single cell experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petzhold, D., da Costa-Goncalves, A.C., Gross, V. et al. Spinophilin is required for normal morphology, Ca2+ homeostasis and contraction but dispensable for β-adrenergic stimulation of adult cardiomyocytes. J Muscle Res Cell Motil 32, 243–248 (2011). https://doi.org/10.1007/s10974-011-9259-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-011-9259-4