Abstract
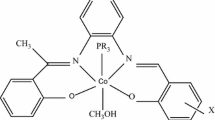
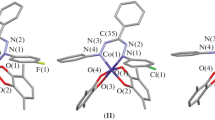
Cobalt(III) complexes of 2,2′-dipyridylamine (dpamH) and the ligands salicylaldehyde (X-saloH) and their corresponding salicylic acids (X-salicylato), where X = CH3, Cl and Br, under the general formula [Co(X-salo)(X-salicylato)(dpamH)] (1–3), were synthesized in situ by slow oxidation in air of ethanolic solutions of the complexes [Co(5-X-salo)2(dpamH)]. The new compounds were characterized by physicochemical methods and by spectroscopy (IR, 1H-NMR and UV–Vis). The octahedral geometry around Co3+ ion and the bidentate chelating mode of the salicylaldehydato anion (X-salo−) and the salicylato di-anion (X-salicylato2−) were proved by single-crystal X-ray diffraction analysis for the complex [Co(5-CH3-salo)(5-CH3-salicylato)(dpamH)] (1). The variable-temperature (76–303 K) magnetic susceptibility measurements showed a diamagnetic nature of the complexes, in accordance with their molecular structure. The simultaneous TG/DTG–DTA technique was used to analyze their thermal behavior under inert and/or oxygen atmosphere, with particular attention to determine their thermal degradation pathways, which was found to have a multi-step nature, accompanied by the release of the ligand molecules.







Similar content being viewed by others
References
Prasad RN, Agrawal A. Synthesis and spectroscopic studies of mixed ligand complexes of cobalt(II) with salicylaldehyde, hydroxyarylketones and beta-diketones. J Indian Chem Soc. 2006;83(1):75–7.
Hussain ST, Ahmad H, Atta MA, Afzal M, Saleem M. High performance liquid chromatography (HPLC), atomic absorption spectroscopy (AAS) and infrared spectroscopy determination and solvent extraction of uranium, using bis(salicylaldehyde) propylene diamine as complexing agent. J Trace Microprobe Tech. 1998;16(2):139–49.
Madan RK, Levitt J. A review of toxicity from topical salicylic acid preparations. J Am Acad Dermatol. 2014;70(4):788–92. doi:10.1016/j.jaad.2013.12.005.
Yiase SG, Adejo SO, Gbertyo JA, Edeh J. Synthesis, characterization and antimicrobial studies of salicylic acid complexes of some transition metals. J Appl Chem (IOSR-JAC). 2014;7(4):4–10.
Tangoulis V, Lalia-Kantouri M, Gdaniec M, Papadopoulos Ch, Miletic V, Czapik A. New type of single chain magnet: pseudo-one-dimensional chain of high-spin Co(II) exhibiting ferromagnetic intrachain interactions. Inorg Chem. 2013;52:6559–69.
Sajith P, Ummer MT, Mandal N, Mandot SK, Agrawal SL, Bandyopadhyay S, Mukhopadhyay R, D’Cruz B, Deuri AS, Kuriakose P. Synthesis of cobalt complexes and their evaluation as an adhesion promoter in a rubber-steel wire system. J Adhes Sci Technol. 2005;19(16):1475–91.
Chen Q. Bis(4-bromo-2-formylphenolato-K2 O, O′)zinc(II). Acta Cryst. 2006;E62(1):m56–7.
Yang Y-M, Lu P-C, Zhu T-T, Liu C-H. Bis(2-formylphenolato-K2 O, O′)iron(II). Acta Cryst. 2007;E63(6):m1613.
Pessoa JC, Cavaco I, Correira I, Tomaz I, Duarte T, Matias PM. Oxovanadium(IV) complexes with aromatic aldehydes. J Inorg Biochem. 2000;80(1):35–9.
Feham K, Benkadari A, Chouaih A, Miloudi A, Boyer G, El Abed D. Synthesis and structural study of triphenylbismuth bis (Salicylate). Cryst Struct Theory Appl. 2013;2:28–33.
Abuhijleh AL. Mononuclear copper(II) salicylate complexes with 1,2-dimethylimidazole and 2-methylimidazole: synthesis, spectroscopic and crystal structure characterization and their superoxide scavenging activities. J Mol Struct. 2010;980:201–7.
Devereux M, McCann M, Casey MT, Martin Curran M, Ferguson G, Cardin Ch, Convery M, Quillet V. Binuclear and polymeric manganese(II) salicylate complexes: synthesis, crystal structure and catalytic activity of [Mn2(Hsal)4(H2O)4] and [{Mn2(sal)2(Hsal)(H2O)–(H3O)(py)4·2py} n ](H2sal = salicylic acid, py = pyridine). J Chem Soc Dalton Trans. 1995;5:771–6.
Larock RC. Comprehensive organic transformations: a guide to functional group preparations. 2nd ed. New York: Wiley; 1999. p. 1653.
Smith MB, March J. Advanced organic chemistry: reactions, mechanisms, and structure. 5th ed. New York: Wiley-Interscience; 2001. p. 917.
Geng J, Tao T, Gu K-H, Wang G, Huang W. Two air oxidation copper(II) complexes of salicylaldehyde derivatives obtained by in situ copper(II) ion catalysis and complexation. Inorg Chem Commun. 2011;14:1978–81.
Papadopoulos Ch, Hatzidimitriou A, Voutsas G, Lalia-Kantouri M. Synthesis and characterization of new addition compounds of bis(substituted-salicylaldehydo) cobalt(II) with 2,2′-bipyridine (bipy). Polyhedron. 2007;26:1077–86.
Papadopoulos CH, Cristóvão B, Ferenc W, Hatzidimitriou A, Vecchio Ciprioti S, Risoluti R, Lalia-Kantouri M. Thermoanalytical, magnetic and structural investigation of neutral Co(II) complexes with 2,2′-dipyridylamine and salicylaldehydes. J Therm Anal Calorim. 2016;123:717–29.
Lalia-Kantouri M, Gdaniec M, Choli-Papadopoulou T, Badounas A, Papadopoulos CD, Czapik A, Geromichalos GD, Sahpazidou D, Tsitouroudi F. Effect of cobalt(II) complexes with dipyridylamine and salicylaldehydes on cultured tumor and non-tumor cells: synthesis, crystal structure investigations and biological activity. J Inorg Biochem. 2012;117:25–34.
Ma P-T, Wang Y-X, Zhang G-Q, Li M-X. Bis(2,2′-bi-pyridyl-κ2N, N′)(carbonato-κ2O, O′)cobalt(III) bromide trihydrate. Acta Cryst. 2008;64:14.
Czapik A, Papadopoulos Ch, Lalia-Kantouri M, Gdaniec M. Carbonato-κ2OO′)bis(di-2-pyridylamine κ2 N, N′)cobalt(III) bromide. Acta Cryst. 2011;E67:m414–5.
De Angelis CS, Kurdziel K, Materazzi S, Vecchio S. Crystal structure and thermoanalytical study of a manganese (II) complex with 1-allylimidazole. J Therm Anal Calorim. 2008;92(1):109–14.
Dziewulska-Kulaczkowska A, Mazur L, Ferenc W. Thermal, spectroscopic and structural studies of zinc(II) complex with Nicotinamide. J Therm Anal Calorim. 2009;96(1):255–60.
Ye HM, Ren N, Li H, Zhang JJ, Sum SJ, Tian L. Synthesis, crystal structure and thermal decomposition kinetics of complex [Nd(BA)3bipy]2. J Therm Anal Calorim. 2010;101(1):205–11.
Madison WI. Bruker analytical x-ray systems, Inc., Apex2, Version 2 User Manual. 2006; M86-E01078.
Palatinus L, Chapuis G. Superflip—a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J Appl Crystallogr. 2007;40:786–90.
Betteridge PW, Carruthers JR, Cooper RI, Prout K, Watkin DJ. Program crystals, software for guided crystal structure analysis. J Appl Crystallogr. 2003;36:1487.
De Meulenaer J, Tompa H. The absorption correction in crystal structure analysis. Acta Cryst. 1965;19(6):1014–8.
Watkin DJ, Prout CK, Pearce LG. CAMERON program, chemical crystallographic laboratory. Oxford: Oxford University; 1996.
Kahn O. Molecular magnetism. New York: VCH Publisher; 1993. p. 38–43.
Figgis BN, Nyholm RS. A convenient solid for calibration of Gouy magnetic susceptibility apparatus. J Chem Soc. 1958;4:4190–1.
Silverstein RM, Bassler GC, Morvill G. Spectrometric identification of organic compounds. 6th ed. New York: Wiley; 1998.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds, part B: applications in coordination, organometallic, and bioinorganic chemistry. 6th ed. New Jersey: Wiley; 2009.
Abuhijleh AL, Woods C. Mononuclear copper (II) salicylate imidazole complexes derived from copper (II) aspirinate. Crystallographic determination of three copper geometries in a unit cell. Inorg Chem Commun. 2001;4(3):119–23.
Tarushi A, Raptopoulou CP, Psycharis V, Terzis A, Psomas G, Kessissoglou DP. Zinc(II) complexes of the second-generation quinolone antibacterial drug enrofloxacin: structure and DNA or albumin interaction. Bioorg Med Chem. 2010;18(7):2678–85.
Ristovic MS, Zianna A, Psomas G, Hatzidimitriou AG, Coutouli-Argyropoulou E, Lalia-Kantouri M. Interaction of dinuclear cadmium(II) 5-Cl-salicylaldehyde complexes with calf-thymus DNA. Mater Sci Eng C. 2016;61:579–90.
Lever AB. Inorganic electronic spectroscopy. 2nd ed. Amsterdam: Elsevier; 1984. p. 462.
Hubin TJ, Alcock NW, Clase HJ, Seib LL, Busch DH. Synthesis, characterization, and X-ray crystal structures of cobalt(II) and cobalt(III) complexes of four topologically constrained tetraazamacrocycles. Inorg Chim Acta. 2002;337:91–102.
Olmez H, Arslam F, Icbudak H. Spectrochemical studies on Co(II), Ni(II), Cu(II) and Zn(II) salicylato (1,10-phenanthroline) complexes. J Therm Anal Calorim. 2004;76(3):793–800.
Ferenc W, Cristvao B, Sarzynski J. Thermal and magnetic behavior of 5-chloro-2-nitrobenzoates of Co(II), Ni(II) and Cu(II). J Therm Anal. 2010;101(2):761–7.
Dziewulska-Kulaczkowska A. Manganese(II), cobalt(II), nickel(II), copper(II) and zinc(II) complexes with 4-oxo-4H-1-benzopyran-3-carboxaldehyde: thermal, spectroscopic and magnetic characterization. J Therm Anal. 2010;101(3):1019–26.
James LE, Crescentini, L, Fisher, WB. Process for making a cobalt oxide catalyst Patent: US 4389339, (A)-1983-06-21.
Acknowledgements
The authors would like to thank Professor Evdoxia Coutouli-Argyropoulou (Aristotle University of Thessaloniki) for recording the 1H–NMR spectra.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10973_2016_5727_MOESM1_ESM.pdf
Detailed crystal data and structure refinement for complex [Co(5–CH3–salo)(5–CH3–salicylato) (dpamH)]. H2O (1) have been deposited with the Cambridge Crystallographic Data Centre under, No CCDC 1472312. Copies of this information may be obtained free of charge from the Director, CCDC, 12 Union Road, Cambridge, CB2 IEZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk). (PDF 160 kb)
Rights and permissions
About this article
Cite this article
Lalia-Kantouri, M., Papadopoulos, C., Hatzidimitriou, A. et al. Oxidized cobalt complexes of salicylaldehydes. J Therm Anal Calorim 126, 1579–1590 (2016). https://doi.org/10.1007/s10973-016-5727-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5727-9