Abstract
The phase equilibria in the DyCl3–LiCl binary system were established on the basis of differential scanning calorimetry (DSC) measurements. One compound, namely Li3DyCl6, is formed in this system. It melts congruently at 768 K with enthalpy of 51.6 kJ mol−1. DSC curves for eutectic composition and for compositions close to eutectic (on the left and right side of eutectic) are identical. Small effect related to the liquidus is shadowed by a big effect related to eutectic, and only one thermal effect is visible. This is the reason that eutectic composition can not be determined directly. The only possibility to determine it precisely is a creation of Tammann diagram. Two eutectics, namely LiCl–Li3DyCl6 and Li3DyCl6–DyCl3, located at DyCl3 mol fractions x = 0.206 (T = 746 K) and x = 0.542 (T = 674 K) were found from Tammann’s plots, which predict, through application of the lever rule, the variation of the enthalpy associated with eutectic melting as a function of composition.
Similar content being viewed by others
Introduction
Rare-earth halides are used in a number of applications such as reprocessing of nuclear wastes, recycling of spent nuclear fuel [1], doses in high-intensity discharge lamps, lasers and new highly efficient light sources with energy-saving features [2, 3]. Technological application of lanthanides halides requires knowledge of their basic thermodynamic properties. These properties are indispensable, e.g., for calculation of the chemical composition in the lamps to enable understanding and hence prediction of tungsten corrosion, silica corrosion and spectra output. The basic thermodynamic properties are also needed for predicting the behavior of doses by modeling of multi-component metal halide systems [4]. However, the available in the literature thermodynamic data on lanthanide halides are very often scarce or contradictory.
Pioneering works devoted to the phase diagrams of lanthanide chloride–alkali metal chloride systems were performed in the sixties of the twentieth century mainly at the Lomonosov University in Moscow [5]. The extensive summaries about chloride systems up to 1977 can be found in the handbook [6] and in the monograph by Prosypajko and Alekseeva [7]. These investigations were accomplished by visual polythermal method. This technique is not a very accurate method and can give quite good results for liquidus lines and thus for existence of congruently melting compounds; however, data on the composition of incongruently melting compounds can be doubtful. Furthermore, solid-state reactions with small reaction enthalpies were often overlooked. As a consequence, all the investigated systems with NaCl were incomplete [5]. Indeed, such a situation could be observed in the case of dysprosium chloride–alkali metal chloride systems. The pioneering work by Korshunov and Drobot [8] demonstrated the existence of Na3DyCl6 and NaDy3Cl10, incongruently melting compounds in the DyCl3–NaCl system, whereas the later work of by Seifert et al. [9] performed by DTA technique showed the existence of three incongruently melting compounds in this system with the following stoichiometry: Na3DyCl6, NaDyCl4 and NaDy2Cl7. Korshunov and Drobot [8] found two congruently melting compounds, namely K3DyCl6 and KDy3Cl10, in the DyCl3–KCl system by visual polythermal method, but Blachnik and Selle [10] demonstrated the existence of three compounds on the basis of DTA measurements. Two of them, namely K3DyCl6 and KDy2Cl7, melt congruently, and K2DyCl5 melts incongruently. The above examples confirm the statement about serious errors and lack of precision of existing phase diagrams of lanthanide chloride–alkali metal chloride binary systems that have been published in the literature. These differences were the cause of systematic reinvestigation of all lanthanide chloride–alkali metal chloride phase diagrams (alkali metal = Na, K, Rb, Cs) that were undertaken by the Seifert’s group from the University of Kassel [5, 11]. Among others, the phase diagrams of the DyCl3–MCl (M = Na, K, Rb, Cs) systems were constructed on the basis of thermal analysis, electrochemical and structural measurements [12]. They confirmed the results obtained by Blachnik and Selle [10] concerning DyCl3–KCl system. Indeed, three compounds were identified in this system. Two of them melt congruently (K3DyCl6 and KDy2Cl7), and the third melts incongruently. As it was stated above, systematic investigations or reinvestigations were performed on the systems of lanthanide chlorides with alkali metal chlorides, with exception of the systems with lithium chloride. Fragmentary information concerning the phase equilibria in the systems of lanthanide chlorides with lithium chloride comes mainly from Chinese journals [13–16]. According to them, the systems with lithium chloride are simple eutectic systems (LaCl3, CeCl3, PrCl3 and NdCl3), systems with LiLn2Cl7 compounds that decompose to a solid-state phase (Ln = Sm, Tb) or systems with Li3LnCl6 compounds that melt incongruently (Ln = Dy, Ho, Er, Yb). According to the literature data [16], the DyCl3–LiCl system is characterized by the existence of Li3DyCl6 compound that undergoes a solid–solid phase transition at 743 K and melts incongruently at 748 K. It should be pointed out that the authors did not inform about a solid–solid phase transition of DyCl3, which is well known from the literature [17]. So, we decided to reinvestigate this system by DSC.
The present work is a part of a multi-instrumental, international program focused on lanthanide halides and their systems with alkali metal halides. The main purpose of this program is to contribute new experimental data concerning unknown systems and verification of the existing data. In the present work, differential scanning calorimetry was used to study thermodynamic properties of DyCl3 and the phase equilibrium in DyCl3–LiCl binary system.
Experimental
Chemicals and samples preparation
Dysprosium(III) chloride was synthesized from dysprosium oxide (99.9 %, Sigma-Aldrich). The first step of this synthesis was dissolution of Dy2O3 in hot concentrated HCl. The obtained solution was evaporated, and DyCl3·6H2O was crystallized. Then ammonium chloride was added, and the obtained mixture of hydrated DyCl3 and NH4Cl was slowly heated up to 1070 K under vacuum in order to remove water and to sublimat unreacted NH4Cl. Finally, the crude DyCl3 was purified by distillation under reduced pressure (~0.1 Pa). The obtained in such a way DyCl3 was of high grade (min. 99.9 %). Chemical analysis was performed by complexometric (dysprosium) and argentometric (chloride) methods. The results were as follows: Dy: 60.40 ± 0.12 % (60.44 % theoretical); Cl: 39.60 ± 0.13 % (39.56 % theoretical). After the synthesis, DyCl3 was ground in a glove box and stored in ampoules sealed under dry argon atmosphere.
The purity of anhydrous lithium chloride was 99.9 % (Alfa Aesar).
The appropriate amounts of DyCl3 and LiCl were melted in vacuum-sealed quartz ampoules in an electric furnace. The melts were homogenized by shaking and solidified. Theses samples were ground in an agate mortar in a glove box. The prepared in this way homogenous mixtures used in measurements focused on phase diagram determination.
Measurements
A Setaram Labsys Evo differential scanning calorimeter (DSC) was used to investigate the phase equilibria in the DyCl3–LiCl system. Experimental samples (100–400 mg) were kept in vacuum-sealed quartz ampoules (about 6 mm diameter, 15 mm length). The DSC experiments on samples with 28 compositions were conducted at heating and cooling rates ranging from 1 to 10 K min−1.
Results and discussion
Phase diagram
DSC investigations performed on 28 samples with different compositions yielded both the temperature and the fusion enthalpy of the concerned mixtures. Due to the supercooling effect observed during cooling runs, all the values of temperature and enthalpy given in this work were determined from the heating curves.
It was established that DyCl3 undergoes a solid–solid phase transition at 606 K and melts at 914 K. These findings are in a good agreement with the literature data [18] that reports 611 K and 919 K for transition and fusion, respectively. Figure 1 shows the DSC curves obtained for the samples with the mole fraction of DyCl3, x = 0.050 (a), 0.250 (b), 0.348 (c), obtained with heating rate of 5 K min−1.
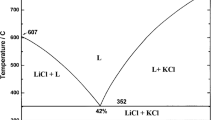
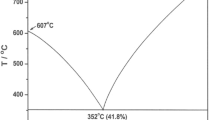
In the composition range 0 < x < 0.250, where x is a mole fraction of DyCl3, two endothermic peaks were present in the all heating DSC curves (Fig. 1a). The effect at the highest temperature corresponds to the liquidus temperature. The second one at 746 K was observed up to x = 0.250, then it disappeared, thus suggesting an existence of a compound with the stoichiometry Li3DyCl6. This thermal feature can be undoubtedly assigned to the LiCl–Li3DyCl6 eutectic. Proper determination of a eutectic composition requires creation of the so-called Tammann diagram. This diagram is an important outcome from the theory of phase diagrams [19–21]. It predicts, through application of the lever rule, the variation of the enthalpy associated with a first-order transformation as a function of concentration. Regrettably, this plot is seldom used or merely overlooked, although it provides valuable information [20]. In order to illustrate its utility, the eutectic melting (Fig. 2) should be considered. For the eutectic mole fraction of component B, x eut, there is only one melting effect corresponding to an enthalpy Δeut H. For other compositions, the enthalpy ΔH given off at the eutectic temperature is proportional to the mole fraction \(x_{\text{eut}}^{*}\) of eutectic liquid formed
Enthalpies should be expressed in joules per mole of sample. Thanks to the lever rule, \(x_{\text{eut}}^{*}\) can be expressed through:
in which x is a mole fraction of component B. Relations (1), (2) and (3) indicate that the variation of ΔH with mole fraction of component B must be linear and going from A to B, ΔH must increase from zero at x = 0 up to Δeut H at x = x eut, and then decrease down to zero at x = 1. Accordingly, x eut can be determined straightforwardly by means of Tammann’s plot. The Tammann plot should start at wherever the left and the right endpoints of the eutectic line are. This means that the plot can give us information on both the eutectic composition and the solid solubilities at eutectic temperature. (The latter are typically the maximum solubilities).
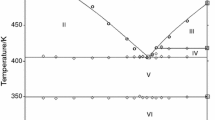
The eutectic contribution to the enthalpy of fusion was determined and plotted against composition in Fig. 3. This Tammann diagram allows to accurately determine the eutectic composition from the intercept of the two linear parts (Fig. 3b), as x = 0.206 ± 0.0086. The mixture with eutectic composition melts with enthalpy, Δfus H m, of about 13.24 ± 1.55 kJ mol−1 at temperature 746 K (a mean value from the experimental values). During construction of the Tammann diagram, it was assumed that there was no solubility in the solid state. Thus, the straight lines intercept the composition axis at x(DyCl3 ) = 0.0 and 0.250.
Only one thermal effect at 768 K was observed on DSC curve for the sample with mole fraction x(DyCl3) = 0.250 (Fig. 1b). It corresponds to congruent melting of Li3DyCl6 compound. The related to this effect molar enthalpy is equal to 51.6 kJ mol−1.
Three thermal effects were observed on the DSC curves for the samples with mole fraction 0.250 < x(DyCl3) < 1.00 (Fig. 1c). The thermal effect at 606 K corresponds to the solid–solid phase transition of DyCl3. The enthalpy values related to the effect at 606 K increase with the increasing mole fraction of DyCl3 (Fig. 3c). The second effect at 674 K (a mean value from experiments) corresponds to Li3DyCl6–DyCl3 eutectic. The eutectic composition x(DyCl3) = 0.542 ± 0.0139 was determined from the Tammann plot displayed in Fig. 3b. The mixture with eutectic composition melts with enthalpy, Δfus H m, of about 11.03 ± 0.49 kJ mol−1 at temperature 674 K. The effect at the highest temperature corresponds to the liquidus temperature.
On the DSC curves, the overlapping of thermal effects related to the eutectic and liquidus was observed, especially for the samples with a mole fraction near to the composition of eutectic (Fig. 4a). Due to this overlapping, it was impossible to determine enthalpy related to the eutectic, which was necessary for Tammann diagrams construction. Thus, it was necessary to perform deconvolution of the overlapping peaks. This separation was made with CALISTO software included in the Labsys Evo differential scanning calorimeter system. The result of application of the peak separation tool is displayed in Fig. 3b. As a result of peaks separation enthalpy related to the eutectics could be determined and used subsequently in the described above Tammann diagrams construction.
All the results of DSC investigations are presented in Table 1, and the complete phase diagram is shown in Fig. 5.
Phase diagram of the DyCl3–LiCl binary system. Values in brackets—experimental data from [16]
The determined by us phase diagram of DyCl3–LiCl binary system is completely different from the one presented in the literature [16]. Although in both cases the existence of Li3DyCl6 compound was confirmed, our findings showed that it melts congruently at 768 K, whereas according to the literature data it melts incongruently at 748 K and additionally undergoes a solid–solid phase transition at 743 K. As a consequence of congruent melting of Li3DyCl6, two eutectics were found by us, namely LiCl–Li3DyCl6 (at x = 0.206 (T eut = 746 K)) and Li3DyCl6–DyCl3 (at x = 0.542 (T = 674 K)). The literature data [16] show only one, Li3DyCl6–DyCl3 eutectic (at x = 0.515 (T = 721 K)). The composition of this eutectic is the only similarity to our results, but its melting temperature is significantly higher than ours (721 and 674 K, respectively). In addition, we found a solid–solid phase transition of DyCl3 in agreement with the literature data [19], whereas this transition is absent in the literature phase diagram [16].
The DyCl3–LiCl system is characterized by the existence of Li3DyCl6 compound. The electrical conductivity of Li3DyCl6 was also measured in the liquid and solid phase. It was found to be a solid electrolyte with a high electrical conductivity at about room temperature.
Conclusions
-
1.
The complete DyCl3–LiCl phase diagram shows the existence of Li3DyCl6 compound that melts congruently at 768 K with enthalpy of 51.6 kJ mol−1.
-
2.
Tammann diagram is an important outcome for the theory of phase diagram. It predicts, through application of the lever rule, the variation of the enthalpy associated with the first-order transformation as a function of concentration. Regrettably, this plot is seldom used or merely overlooked, although it provides valuable information.
-
3.
Tammann diagrams construction allows to precisely determine the composition of eutectics existing in the system.
-
4.
The composition and temperature of eutectics were determined as x(DyCl3) = 0.206; T eut = 746 K and x(DyCl3) = 0.542; T eut = 674 K.
References
Naumov VS, Bychkov AV, Lebedev A. Advenctes in molten salts: from structural aspects to waste processing. In: Gaude-Escard M, editor. Properties of liquid-salt nuclear fuel and its reprocessing technology. Danbury: Begell House Inc; 1999. p. 432–53.
Junming T, Bath NY. Quarty metal halide lamp with improved lumen maintenance. U.S. Patent Application Publication. 2008; US2008/0093993 Ai.
Junming T, Bath NY. Quarty metal halide lamp with improved lumen maintenance. U.S. Patent Application Publication. 2010; US 7, 786,674 B2.
Guest EC, Mucklejohn SA, Preston B, Rouffet JB, Zissis G., NumeLiTe: an energy effective lighting system for roadways and an industrial application of molten salts. In: Oye HA, Jagtoyen A editors, Proceedings of International Symposium on Ionic Liquids in Honour of M. Gaune-Escard, Carry le Rouet, France 2003; 26–28: pp. 37-45.
Seifert HJ. Ternary chlorides of the trivalent late lanthanides: phase diagrams, crystal structures and thermodynamic properties. J Therm Anal Calorim. 2006;83:479–505.
Gmelin L. Handbook of Inorganic Chemistry. 8th ed., vol. C5. Berlin: Springer; 1977.
Prosypajko VI, Alekseeva EA. Phase equilibria in binary halides. In: Bell HB, editor. New York: IFI/Plenum.
Korshunov BG, Drobot DV. Interaction of gadolinium and dysprosium chlorides with chlorides of sodium and potassium (in Russian). Zh Neorg Khimii. 1965;10:939–42.
Seifert HJ, Sandrok J, Uebach J. Thermochemical and structural investigations on the systems NaCl/TbCl3 and NaCl/DyCl3. Acta Chemica Scandavica. 1995;49:653–7.
Blachnik R, Selle D. Zur Thermochemie von Alkalichlorid-Lanthanoid(III)-chloriden. Z Anorg Allg Chem. 1979;454:90–8.
Seifert HJ. Ternary chlorides of the trivalent early lanthanides: phase diagrams, crystal structures and thermodynamic properties. J Therm Anal Calorim. 2002;67:789–826.
Seifert HJ, Krämer R. Ternare chloride in den systemen ACl/DyCl3 (A = Cs, Rb, K). Z Anorg Allg Chem. 1994;620:1543–8.
Chao-Gui Zheng, Shou-Lin Huang, Si-Qiang Wang. Phase diagram of binary systems ErCl3–MCln (M = Li, Ca, Pb; n = 1 or 2). Chem J Chin Univ. 1993;14:992–5.
Chao-Gui Zheng, Shou-Lin Huang, Si-Qiang Wan. Phase Diagram of Binary Systems YbCl3–MCln (M = Li, Mg, Ca, Pb; n = 1 or 2). Acta Phys Chim Sin. 1994;10:342–7.
Chao-Gui Zheng, Shou-Lin Huang, Si-Qiang Wang. Phase diagram of binary system HoCl3–MCln (M = Li, Mg, Ca, Pb; n = 1 or 2). Acta Chim Sinica. 1994;52:735–9.
Chao-Gui Zheng, Zhong-Dong Zhao, Si-Qiang Wang. Phase diagram of binary systems DyCl3–MCl (M = Li, Mg, Ca, Pb; n = 1 or 2). Inst Sci Tech Inf China. 1994;18:263.
Gaune-Escard M, Rycerz L, Szczepaniak W, Bogacz A. Enthalpies of phase transition in the lanthanide chlorides LaCl3, CeCl3, PrCl3, NdCl3, GdCl3, DyCl3, ErCl3 and TmCl3. J Alloys Comp. 1994;204:193–6.
Rycerz L. Thermochemistry of lanthanide halides and compounds formed in lanthanide halide–alkali metal halide systems (in Polish). Scientific Papers of Institute of Inorganic Chemistry and Metallurgy of Rare Elements. Wroclaw University of Technology. 2004;35.
Findlay A. The phase rule and its applications. New York: Longmans, Green and Co.; 1911.
Guenet JM. Contributions of phase diagrams to the understanding of organized polymer-solvent systems. Thermochim Acta. 1996;284:67–83.
Rycerz L. Practical remarks concerning phase diagram determination on the base of DSC measurements. J Therm Anal Cal. 2013;113:231–8.
Acknowledgements
The work was financed by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry of Wroclaw University of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dańczak, A., Rycerz, L. Reinvestigation of the DyCl3–LiCl binary system phase diagram. J Therm Anal Calorim 126, 299–305 (2016). https://doi.org/10.1007/s10973-016-5547-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5547-y