Abstract
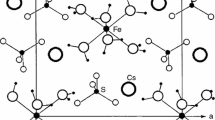
The structural and thermodynamic properties of Tutton salt K2Zn(SO4)2·6H2O were investigated using thermogravimetric analysis, differential scanning calorimetry, and nuclear magnetic resonance. The first mass loss of H2O occurred around 353 K, which was interpreted as the onset of partial thermal decomposition, and the mass loss continues from T d to 440 K. The temperature dependences of the spin–lattice relaxation time for the 1H and 39K nuclei were measured in the laboratory frame and in the rotating frame. The results were compared with those obtained for the series of compounds, i.e., M2Zn(SO4)2·6H2O (M = Na, Rb, and Cs), that were previously reported.








Similar content being viewed by others
References
Gronvold F, Meisingset KK. Thermodynamic properties and phase transitions of salt hydrates between 270 and 400 K I. NH4Al(SO4)2·12H2O, KAl(SO4)2·12H2O, Al2(SO4)3·17H2O, ZnSO4·7H2O, Na2SO4·10H2O, and Na2S2O3·5H2O. J Chem Therm. 1982;14:1083–98.
Lim AR. Study on the phase transitions by nuclear magnetic resonance of α-type RbAl(SO4)2·12H2O and β-type CsAl(SO4)2·12H2O single crystals. Solid State Nucl Magn Reson. 2009;36:45–51.
Jain VK, Venkateswarlu P. On the 57Fe Mossbauer spectra of FeTe and Fe2Te3. J Phys C Solid State Phys. 1979;12:865–73.
Marinova D, Georgiev M, Stoilova D. Vibrational behavior of matrix-isolated ions in Tutton compounds. II. Infrared spectroscopic study of NH4 + and SO4 2− ions included in copper sulfates and selenates. J Mol Struct. 2009;938:179–84.
Marinova D, Georgiev M, Stoilova D. Vibrational behavior of matrix-isolated ions in Tutton compounds. I. Infrared spectroscopic study of NH4 + and SO4 2− ions included in magnesium sulfates and selenates. J Mol Struct. 2009;929:67–72.
Marinova D, Georgiev M, Stoilova D. Vibrational behavior of matrix-isolated ions in Tutton compounds. IV. Infrared spectroscopic study of NH4 + and SO4 2− ions included in nickel sulfates and selenates. Cryst Res Technol. 2010;45:637–42.
Georgiev M, Marinova D, Stoilova D. Vibrational behavior of matrix-isolated ions in Tutton compounds. III. Infrared spectroscopic study of NH4 + and SO4 2− ions included in cobalt sulfates and selenates. Vib Spectrosc. 2010;53:233–8.
Riley MJ, Hitchman MA, Mohammed AW. Interpretation of the temperature dependent g values of the Cu(H2O) 2+6 ion in several host lattices using a dynamic vibronic coupling model. J Chem Phys. 1987;87:3766–78.
Hoffmann SK, Goslar J, Hilczer W, Augustyniak MA, Marciniak M. Vibronic behavior and electron spin relaxation of Jahn–Teller complex Cu(H2O) 2+6 in (NH4)2Mg(SO4)2·6H2O single crystal. J Phys Chem A. 1998;102:1697–707.
Parthiban S, Anandalakshmi H, Senthilkumar S, Karthikeyan V, Mojumdar SC. Influence of Vo(II) doping on the thermal and optical properties of magnesium rubidium sulfate hexahydrate crystals. J Therm Anal Calorim. 2012;108:881–5.
Ganesh G, Ramadoss A, Kannan PS, Subbiahpandi A. Crystal growth, structural, thermal, and dielectric characterization of Tutton salt (NH4)2Fe(SO4)2·6H2O crystals. J Therm Anal Calorim. 2013;112:547–54.
Montgomery H, Lingafelter EC. The crystal structure of Tutton’s salts. III. Copper ammonium sulfate hexahydrate. Acta Crystallogr. 1966;20:659–62.
SMART and SAINT-Plus v6.22 (2000) Bruker AXS Inc., Madison, Wisconsin, USA.
Cowan B. Nuclear Magnetic Resonance and Relaxation. UK: Cambridge University; 1997.
Dolinsek J, Arcon D, Zalar B, Pire R, Blinc R, Kind R. Quantum effects in the dynamics of proton glasses. Phys Rev B. 1996;54:R6811–4.
Lim AR, Lee JH. 23Na and 87Rb relaxation study of the structural phase transitions in the Tutton salts Na2Zn(SO4)2·6H2O and Rb2Zn(SO4)2·6H2O single crystals. Phys Status Solidi B. 2010;247:1242–6.
Lim AR, Kim SH. Study on structural phase transitions and relaxation processes of Cs2Co(SO4)2·6H2O and Cs2Zn(SO4)2·6H2O crystals. Mater Chem Phys. 2009;117:557–61.
Lee KS. Comments concerning “Thermodynamic properties and phase transitions of Tutton salt (NH4)2Co(SO4)2·6H2O crystals”. J Therm Anal Calorim. 2014;115:975–7.
Acknowledgements
This research was supported by the Basic Science Research program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2015R1A1A3A04001077).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, A.R., Kim, S.H. Structural and thermodynamic properties of Tutton salt K2Zn(SO4)2·6H2O. J Therm Anal Calorim 123, 371–376 (2016). https://doi.org/10.1007/s10973-015-4865-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4865-9