Abstract
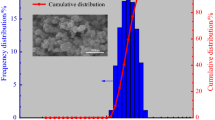
Owing to increasing threats of biological attacks, new methods for the neutralization of spore-forming bacteria are currently being examined. Thermites may be an effective method to produce high-temperature reactions, and some compositions such as aluminum (Al) and iodine pentoxide (I2O5) also have biocidal properties. This study examines the thermal degradation behavior of I2O5 mixed with micron and nanometer scale aluminum (Al) particles. Differential scanning calorimetry (DSC) and thermogravimetric (TG) analyses were performed in an argon environment on both particle scales revealing a non-reaction for micron Al and a complex multistep reaction for the nanometer scale Al. Results show that upon I2O5 decomposition, iodine ion sorption into the alumina shell passivating Al particles is the rate-controlling step of the Al–I2O5 reaction. This pre-ignition reaction is unique to nano-Al mixtures and attributed to the significantly higher specific surface area of the nanometric Al particles which provide increased sites for I− sorption. A similar pre-ignition reaction had previously been observed with fluoride ions and the alumina shell passivating Al particles.




Similar content being viewed by others
References
Harigel GG. History of biological, chemical, and radiation emergencies. Conference on Biosecurity and Bioterrorism, 2000. p. 1–18.
Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Hauer J, McDade J, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Tonat K. Anthrax as a biological weapon. J Am Med Assoc. 1999;281:1736–45.
Blatchley ER, Meeusen A, Aronson AI, Brewster L. Inactivation of Bacillus spores by ultraviolet or gamma radiation. J Environ Eng. 2005;131(9):1245.
Babaitsev IV, Arzhevitov SY, Presnakova OA. Evaluating blast pressure of iron-aluminum thermites. Metallurgist. 2007;51(7–8):348–350.
Dvoryankin AV, Strunina AG, Merzhanov AG. Trends in the spin combustion of thermites. Combust Explos Shock Waves. 1982;18(2):75–79.
Taylor SL, Fina LR, Lambert JL. New water disinfectant: an insoluble quaternary ammonium resin-triiodide combination that releases bactericide on demand. Appl Environ Microbiol. 1970;20(5):720–2.
Wiberg E, Wiberg N, Holleman AF, editors. Inorganic chemistry. 1st ed. 2001, p. 465.
Osborne DT, Pantoya ML. Effect of particle size on the thermal degradation of Al/teflon mixtures. Combust Sci Technol. 2007;179(8):1467–80.
Sarbak Z. Effect of fluoride and sodium ions on structural and thermal properties of gamma-Al2O3. Cryst Res Technol. 1997;32(4):491–7.
Toyohara M, Kaneko M, Mitsutsuka N, Fujihara H, Saito N, Murase T. Contribution to understanding iodine sorption mechanism onto mixed solid alumina cement and calcium compounds. J Nucl Sci Technol. 2002;39(9):950–6.
Trunov MA, Schoenitz M, Xiaoying Z, Dreizin EL. Effect of polymorphic phase transformations in Al2O3 film on oxidation kinetics of aluminum powders. Combust Flame. 2005;140:310–8.
Granier JJ, Pantoya ML. Laser ignition of nanocomposite thermites. Combust Flame. 2004;138(4):373–83.
Dean SW, Pantoya ML. The influence of alumina passivation on nano-Al/Teflon reactions. Thermochimica Acta. 2009;493(1–2):109–10.
Acknowledgements
The authors gratefully acknowledge the support from the Defense Threat Reduction Agency (DTRA) on this project, and the interest and encouragement from our program manager, Dr. Suhithi Peiris.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farley, C., Pantoya, M. Reaction kinetics of nanometric aluminum and iodine pentoxide. J Therm Anal Calorim 102, 609–613 (2010). https://doi.org/10.1007/s10973-010-0915-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0915-5