Abstract
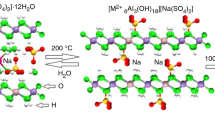
Zn-Al hydrotalcites and Cu-Al hydrotalcites were synthesised by coprecipitation method and analysed by X-ray diffraction (XRD) and thermal analysis coupled with mass spectroscopy. These methods provide a measure of the thermal stability of the hydrotalcite. The XRD patterns demonstrate similar patterns to that of the reference patterns but present impurities attributed to Zn(OH)2 and Cu(OH)2. The analysis shows that the d003 peak for the Zn-Al hydrotalcite gives a spacing in the interlayer of 7.59 Å and the estimation of the particle size by using the Debye-Scherrer equation and the width of the d003 peak is 590 Å. In the case of the Cu-Al hydrotalcite, the d003 spacing is 7.57 Å and the size of the diffracting particles was determined to be 225 Å.
The thermal decomposition steps can be broken down into 4 sections for both of these hydrotalcites. The first step decomposition below 100°C is caused by the dehydration of some water absorbed. The second stage shows two major steps attributed to the dehydroxylation of the hydrotalcite. In the next stage, the gas CO2 is liberated over a temperature range of 150°C. The last reactions occur over 400°C and involved CO2 evolution in the decomposition of the compounds produced during the dehydroxylation of the hydrotalcite.
Similar content being viewed by others
References
R. M. Taylor, Clay Miner., 17 (1982) 369.
H. F. W. Taylor, Miner. Mag. J. Miner. Soc., (1876–1968) 37 (1969) 338–342.
H. C. B. Hansen and C. B. Koch, Appl. Clay Sci., 10 (1995) 5.
Y.-H. Lin, M. O. Adebajo, R. L. Frost and J. T. Kloprogge, J. Therm. Anal. Cal., 81 (2005) 83.
Y.-H. Lin, M. O. Adebajo, J. T. Kloprogge, W. N. Martens and R. L. Frost, Mater. Chem. Phys., 100 (2006) 174.
R. L. Frost and K. L. Erickson, J. Therm. Anal. Cal., 76 (2004) 217.
R. L. Frost and M. L. Weier, Thermochim. Acta, 409 (2004) 79.
R. L. Frost, M. L. Weier and K. L. Erickson, J. Therm. Anal. Cal., 76 (2004) 1025.
R. Allmann and J. D. H. Donnay, Am. Mineral., 54 (1969) 296.
T. Moroz, L. Razvorotneva, T. Grigorieva, M. Mazurov, D. Arkhipenko and V. Prugov, Appl. Clay Sci., 18 (2001) 29.
J. T. Kloprogge and R. L. Frost, Appl. Catal., A: General, 184 (1999) 61.
A. Alejandre, F. Medina, X. Rodriguez, P. Salagre, Y. Cesteros and J. E. Sueiras, Appl. Catal., B 30 (2001) 195.
J. Das and K. Parida, React. Kinet. Catal. Lett., 69 (2000) 223.
S. H. Patel, M. Xanthos, J. Grenci and P. B. Klepak, J. Vinyl Addit. Technol., 1 (1995) 201.
V. Rives, F. M. Labajos, R. Trujillano, E. Romeo, C. Royo and A. Monzon, Appl. Clay Sci., 13 (1998) 363.
F. Rey, V. Fornes and J. M. Rojo, J. Chem. Soc., Faraday Trans., 88 (1992) 2233.
M. Valcheva-Traykova, N. Davidova and A. Weiss, J. Mater. Sci., 28 (1993) 2157.
R. L. Frost, J. M. Bouzaid, A. W. Musumeci, J. T. Kloprogge and W. N. Martens, J. Therm. Anal. Cal., 86 (2006) 437.
R. L. Frost and Z. Ding, Thermochim. Acta, 405 (2003) 207.
R. L. Frost and K. L. Erickson, Thermochim. Acta, 421 (2004) 51.
R. L. Frost, W. Martens, Z. Ding and J. T. Kloprogge, J. Therm. Anal. Cal., 71 (2003) 429.
R. L. Frost, A. W. Musumeci, T. Bostrom, M. O. Adebajo, M. L. Weier and W. Martens, Thermochim. Acta, 429 (2005) 179.
R. L. Frost, A. W. Musumeci, J. T. Kloprogge, M. L. Weier, M. O. Adebajo and W. Martens, J. Therm. Anal. Cal., 86 (2006) 205.
R. L. Frost, M. L. Weier, M. E. Clissold, P. A. Williams and J. T. Kloprogge, Thermochim. Acta, 407 (2003) 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Voyer, N., Soisnard, A., Palmer, S.J. et al. Thermal decomposition of the layered double hydroxides of formula Cu6Al2(OH)16CO3 and Zn6Al2(OH)16CO3 . J Therm Anal Calorim 96, 481–485 (2009). https://doi.org/10.1007/s10973-008-9169-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-008-9169-x