Abstract
Lanthanum ferrite is reported to possess catalytic properties. However, catalytic activity of Mn-doped lanthanum ferrites has not been reported so far. The work reports, for the first time, the synthesis of LaMn x Fe1−x O3 (x = 0.0, 0.1, 0.2, 0.3, 0.4 and 0.5) and investigates their catalytic activity in the presence and absence of visible light irradiation. The synthesized ferrites possessed phase purity and had same symmetry as pure LaFeO3. The unit cell volume of ferrite compositions decreased with increasing manganese content. The average crystallite size was ~60 nm. The d.c. resistivity of ferrites decreased with increasing temperature, confirming semiconductor behaviour of ferrite compositions. Further, these were utilized to study the decomposition reaction of hydrogen peroxide solution. It was observed that the ferrites catalysed the decomposition of hydrogen peroxide. The rate of hydrogen peroxide decomposition increased with increasing manganese substitution. Based upon this observation, the ferrites were employed as heterogeneous catalysts in the H2O2-assisted degradation of anionic dyes (Remazol Turquoise Blue, Remazol Brilliant Yellow) and cationic dyes (Methylene Blue, Safranine-O) in absence as well as in the presence of visible light irradiation.
Graphical Abstract
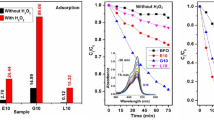
The hydrogen peroxide decomposition using different types of ferrites as heterogeneous catalysis was studied. The catalytic activity was found to be strongly dependent on the particle size and the cation distribution of the ferrite sample.












Similar content being viewed by others
References
Mih NQ (1993) Ceramic fuel cells. J Am Ceram Soc 76(3):563–588
Delmastro A, Mazza D, Ronchetti S (2001) Synthesis and characterization of non stoichiometric LaFeO3 perovskite. Mater Sci Eng B Solid 79:140–145
Ge X, Liu Y, Liu X (2001) Preparation and gas sensitive properties of LaFe1-yCOyO3 semiconducting materials. Sens Actuators, B 79:171–174
Hammami R, Batis H, Minot C (2009) Combined experimental and theoretical investigation of the CO2 adsorption on LaMnO3+y perovskite oxide. Surf Sci 603:3057–3067
Han LA, Chen CL, Dong HY, Wang JY, Gao GM (2008) Effect of Al doping on the magnetic and electrical properties of layered perovskite La1.3Sr1.7Mn2xAlxO7. Phys B 403:2614–2617
Poplawski K, Lichtenberger J, Keil FJ, Schnitzlein K, Amiridis MD (2000) Catalytic oxidation of 1,2-dichlorobenzene over ABO3-type perovskites. Catal Today 62:329–336
Fergus JW (2006) Oxide anode materials for solid oxide fuel cells. Solid State Ionics 177:1529–1541
Alcock CB, Doshi RC, Shen Y (1992) Perovskite electrodes for sensors. Solid State Ionics 51:281–289
Antunes AB, Gilc V, Mourec C, Pena O (2007) Magnetic properties of Er(Co, Mn)O3 perovskites. J Eur Ceram Soc 27:3927–3930
Casbeer E, Sharma VK, Li XZ (2012) Synthesis and photocatalytic activity of ferrites under visible light: a review. Sep Purif Technol 87:1–14
Guan YH, Ma J, Ren YM, Liu YL, Xiao JY, Lin LQ, Zhang C (2013) Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals. Water Res 47:5431–5438
Hu R, Li C, Wang X, Sun Y, Jia H, Su H, Chang Y (2012) Photocatalytic activities of LaFeO3 and La2FeTiO6 in p-chlorophenol degradation under visible light. Catal Commun 29:35–39
Wei ZX, Wang Y, Liu JP, Xiao CM, Zeng WW (2012) Synthesis, magnetization and photocatalytic activity of LaFeO3 and LaFe0.5Mn0.5-xO3-δ. Mater Chem Phys 136:755–761
Li S, Jing L, Fu W, Yang L, Xin B, Fu H (2007) Photoinducedcharge property of nanosized perovskite-type LaFeO3and its relationship with photocatalytic activity under visible light irradiation. Mater Res Bull 42:203–212
Tang P, Tong Y, Chen H, Cao F, Pan G (2013) Microwave-assisted synthesis of nanoparticulate perovskite LaFeO3 as a high active visible-light photocatalyst. Curr Appl Phys 13:340–343
Rusevova K, Koferstein R, Rossel M, Richnow HH, Kopinke FD, Georgi A (2014) LaFeO3 and BiFeO3 perovskites as nanocatalysts for contaminant degradation in heterogeneous Fenton-like reactions. Chem Eng J 239:322–331
Khataee AR, Kasiri MB (2010) Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide: influence of the chemical structure of dyes. J Mol Catal A: Chem 328:8–26
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Int 30:953–971
Chacko JT, Subramanium K (2011) Enzymatic degradation of azo dyes—a review. Int J Environ Sci 1:1250–1260
Soltani T, Entezari M (2013) Sono-synthesis of bismuth ferrite nanoparticles with high photocatalytic activity in degradation of Rhodamine B under solid light irradiation. Chem Eng J 223:145–154
Sathishkumar P, Mangalaraja RV, Anandan S, Kumar MA (2013) CoFe2O4/TiO2 nanocatalysts for photocatalytic degradation of Reactive Red 120 in aqueous solution in the presence and absence of electron acceptors. Chem Eng J 220:302–310
Mahmoodi NM (2013) Zinc ferrite nanoparticles as a magnetic catalyst: synthesis and dye degradation. Mater Res Bull 48:4255–4260
Ahmed MA, Seouidi R, El-dek SI (2005) Spectroscopic and structural analysis of Ca substituted La orthoferrite. J Mol Struct 754:41–44
Rao GVS, Rao CNR, Ferraro JR (1970) Infrared and electronic spectra of rare earth perovskites: ortho-chromites, -magnetites and -ferrites. Appl Spectrosc 24:436–445
Culity BD (1976) Elements of X-ray diffraction, chapter 14. Addison-Wesly Publishing Co. Inc., Reading, MA
Iqbal MJ, Siddiquah MR (2008) Electrical and magnetic properties of chromium-substituted cobalt ferrite nanomaterials. J Alloys Compd 453:513–518
Ajmal M, Shah NA, Maqsood A, Awan MS, Arif M (2010) Influence of sintering time on the structural, electrical and magnetic properties of polycrystalline Cu0.6Zn0.4Fe2O4 ferrites. J Alloys Compd 508:226–232
Ashiq MN, Bibi N, Malana MA (2010) Effect of Sn–Ni substitution on the structural, electrical and magnetic properties of mixed spinel ferrites. J Alloys Compd 490:594–597
Lin SS, Gurol MD (1998) Catalytic decomposition of hydrogen peroxide on iron oxide: kinetics, mechanism, and implications. Environ Sci Technol 32:1417–1423
Nagaraja S, Kottam N, Girija CR, Nagabhushana BM (2012) Photocatalytic degradation of Rhodamine B dye under UV/solar light using ZnO nanopowder synthesized by solution combustion route. Powder Technol 215–216:91–97
Kalsoom U, Ashraf SS, Meetani MA, Rauf MA, Bhatti HN (2013) Mechanistic study of a diazo dye degradation by Soybean Peroxidase. Chem Cent J 7:93–103
Acknowledgments
The authors express their deep gratitude to the University Grants Commission (UGC) and Department of Science and Technology (DST) for providing financial assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jauhar, S., Dhiman, M., Bansal, S. et al. Mn3+ ion in perovskite lattice: a potential Fenton’s reagent exhibiting remarkably enhanced degradation of cationic and anionic dyes. J Sol-Gel Sci Technol 75, 124–133 (2015). https://doi.org/10.1007/s10971-015-3682-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3682-8